Edited by Jacy Reese Anthis.
Working draft. Last updated June 17, 2019.
Abstract
This report seeks to understand the choices and strategies that can hasten or hurt the adoption of novel food technologies by examining how genetically modified (GM) food became an object of controversy in the United States and Europe. Among other conclusions, this report finds that perceptions of food companies as secretive and aggressive damaged GM food adoption, that GM firms understood their work to be humanitarian, innovative, and environmentally-friendly and so were largely caught unawares by popular backlash, that technology adoption is more readily affected by advocacy when buyers in a supply chain exert relatively more pressure on sellers than the reverse, and that focusing on the positive aspects of a technology has been more successful for encouraging its adoption than focusing on responding to negative perceptions.
Table of Contents
Introduction
The minute they tell you not to worry about something, you worry.
— North London woman on GMOs, 1996[1]
The use of genetically modified organisms (GMOs) in food[2] has been riddled with controversy for five decades, including various cases of adoption and rejection that coincide with a range of messaging and activism. Studying this history can yield useful conclusions for predicting the adoption of other potentially controversial food technologies. Of course, the comparisons need to be accompanied by discussion of the relevant analogies and disanalogies.
I begin with an outline of genetic engineering technology and the route GM foods have taken to acceptance or rejection in US and EU markets, since these have been the main battlegrounds of GM adoption. The bulk of the report will discuss various analogies and disanalogies between GM food adoption and the case of clean meat, a nascent food technology that could face similar controversies. Finally, I will summarize the implications and major findings of the report.
This case study doesn't argue for or against GM food. We’re interested in how GM foods became an object of controversy and how this has affected their adoption. We’re looking for strategic lessons for people who are working on the adoption of new, potentially controversial technologies like clean and plant-based meat—as well as, to a lesser extent, lessons for people working to oppose similar technologies.
Overall, this report provides evidence for the following claims:
- Developers of emerging technologies should avoid being unduly secretive, aggressive, or arrogant—or even being perceived this way by activists or the general public.
- Even if the developers of a technology are transparent and socially conscious when the technology first emerges (as clean meat advocates are today), there is still significant risk of negative perception down the road. The first GMO firms to market like Calgene and Zeneca were also relatively transparent and socially conscious, yet those firms merged with or were acquired by larger firms. Moreover, even the developers at those larger firms saw themselves as innovators and humanitarians, suggesting that much caution is needed with emerging technology even if one has the best intentions.
- Activists should focus more on relatively small campaigns, especially those that pressure companies occupying vulnerable positions in a supply chain, rather than large campaigns that rely on shifting public opinion. Much of the successful activist action against GM food came in this form.
- When buyers can exert more pressure on sellers than the reverse, technology adoption is more readily affected by advocacy because buyers and firms further down the supply chain are more susceptible to consumer pressure.
- It is often difficult to convince emerging technology firms to use effective strategies, even though the effective strategies can be relatively easy to figure out and implementing them is in the long-term best interests of the nascent industry.
- No single feature of a technology is sufficient to ensure or prohibit adoption (e.g. being perceived as unnatural). Technology advocates shouldn’t put all their resources into a single issue, even if it’s the most important issue.
- Focusing on the positive aspects of a technology has been more successful for encouraging its adoption than focusing on responding to negative perceptions.[3] Constant discussion of safety concerns, even if to answer these concerns in a technically-sound manner, tends to displace positive framing of an issue and reinforce the idea that there is something to fear. This dynamic is exacerbated by the fact that non-experts often make decisions based on acceptability rather than risk, so a technical totting-up of the relative risks and benefits of a technology is likely to be subsumed in public discussion to a reactive acceptability/nonacceptability binary.
Further implications and findings are described below.
How did GM food come to be?
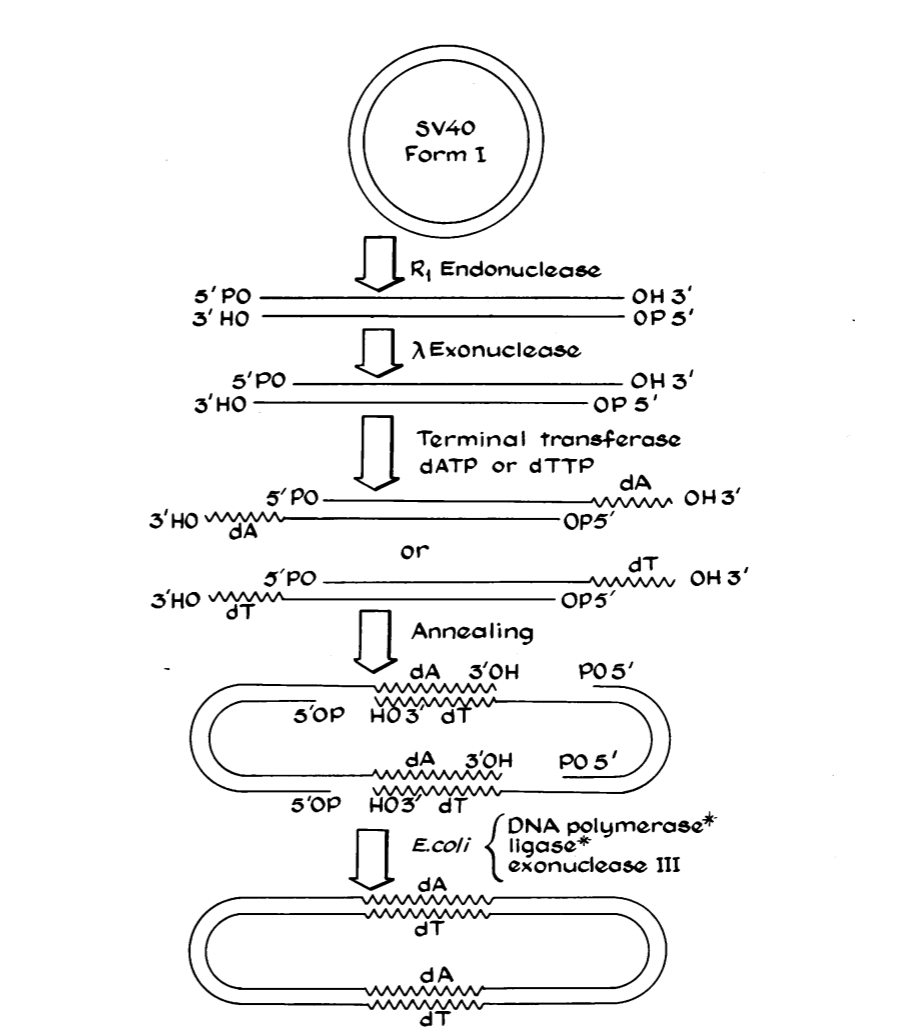
Modern genetic engineering began in 1972 when biochemist Paul Berg opened a loop of simian virus DNA, inserted genes from Enterobacteria phage λ, and reclosed the monkey virus’s dimer circle with part of the lambda phage’s DNA inside.[4]
Fig. 1. Berg’s original method for combining the DNA of two different viruses. Berg, “Biochemical Method,” 2905.
In 1973, Herbert Boyer and Stanley Cohen spliced a variety of genes into E. coli, including genes that endowed the altered bacteria with certain types of antibiotic resistance and genes from the toad Xenopus laevis (a common model organism).[5]
Safety concerns accompanied recombinant DNA research from the beginning. Paul Berg had originally intended to re-insert his hybrid simian virus/lambda virus DNA into E. coli, but did not carry out this step due to fears that the altered form of E. coli might spread to humans.[6] In 1975, Berg organized the Asilomar Conference on Recombinant DNA, a meeting of about 140 scientists, lawyers, and doctors that put forward voluntary but influential guidelines on rDNA research. These guidelines included steps like building containment procedures directly into experimental design.[7]
By 1976, Boyer started Genentech, widely recognized as the first genetic engineering (GE) company, with venture capital funding. By 1977, the firm had inserted genes for insulin production into E. coli.[8] Five years later, the FDA approved Humulin, a form of synthetic insulin pioneered by Genentech. Today, GM strains of yeast or E. coli produce most of the world’s insulin, making insulin more widely available for diabetics.[9]
Research into transgenic food began in the 1970s and by 1982 had produced the first transgenic plant, a tobacco plant resistant to the antibiotic kanamycin.[10]
GM crops were not commercialized until 1992, when Chinese farmers planted strains of virus-resistant tobacco.[11] GM tobacco was pulled from China between 1995 and 1997 after tobacco buyers, especially US cigarette manufacturers, worried that consumers would reject GM tobacco.
The first commercially-available GM food, Calgene’s Flavr Savr tomato, incorporated a gene that slowed pectin degradation and therefore extended the tomato’s shelf life. Calgene introduced the tomato in May of 1994. Despite pushback from early anti-GMO activists like Jeremy Rifkin, the Flavr Savr remained in demand. Calgene employed positive labeling and transparency in its branding, using “label[s] on the cellophane wrapper on the tomato” and distributing “point of purchase brochures explaining how the tomato was genetically engineered.”[12] The tomato packaging displayed a 1-800 number inviting customers to call with questions:
Fig. 2. Flavr Savr packaging in the mid-1990s. See Michael Winerip, “You Call That a Tomato?” New York Times, June 24, 2013, http://www.nytimes.com/2013/06/24/booming/you-call-that-a-tomato.html.
However, Calgene, which had never been in the business of fruit distribution, struggled to lower production costs. The company—mostly run by self-described “gene jockeys,” not farmers—made a number of elementary errors, e.g. destroying shipments by failing to pack trucks correctly. “Uh, we had to get a lot of the fruit out by shovel,” Bill Hiatt, former VP of Research and Development at Calgene, admitted to the New York Times in 2013. Flavr Savr tomatoes never became profitable. Monsanto purchased Calgene on May 21, 1997, the third anniversary of the introduction of the Flavr Savr.
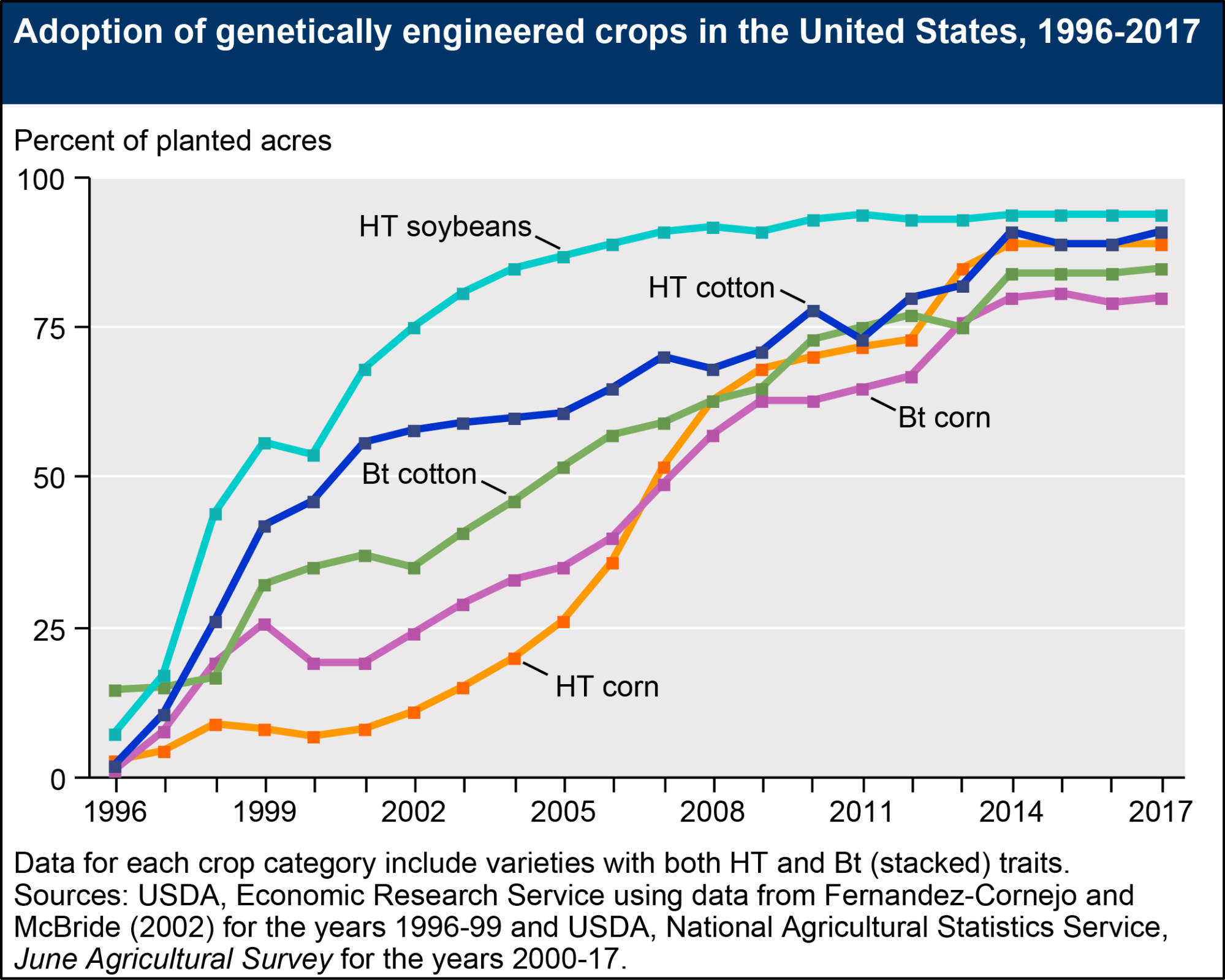
As of 2016, twenty-six countries actively plant GM crops. The US, with 39% of global GM planting by area, leads the world. Brazil (27%), Argentina (13%), Canada (6%), and India (6%) follow.[13] About 86% of US planting by area is GM. Soybeans account for half of all GM acreage, followed by corn (33%), cotton (12%), and canola (5%). Virtually all US adoption has come between 1996 and 2014.
Fig. 3. US GE adoption by crop. “HT” refers to herbicide tolerant strains and “Bt” to strains that produce insecticidal proteins from Bacillus thuringiensis, a soil bacterium known for parasitizing a variety of insects. ERS, 2017, https://www.ers.usda.gov/data-products/adoption-of-genetically-engineered-crops-in-the-us/recent-trends-in-ge-adoption.aspx.
However, the US adoption of GM crops should be characterized as a crop-by-crop phenomenon. Wheat, rice, potatoes, melons, and tomatoes all remain unplanted in the United States, despite successful tests or even brief commercialization (including, in the case of the Flavr Savr tomato and NewLeaf potato, reasonably widespread consumption). Most of these retreats came about as some variant of situations in which, as in the case of the GM potato, “foodservice chains [e.g. chain restaurants and companies running school and hospital cafeterias] told farmers they worried about campaigns portraying their french fries as made of GMOs.”[14] Resistance to GE technology had existed from before the 1975 Asilomar Conference, and early activists like Jeremy Rifkin had established criticisms of GMOs dating to the 1970s, but these concerns failed to show up in wider public opinion polling and consumption patterns through the mid-1990s. Public opposition to GMOs would rise in the late 1990s (probably due in part to increased public exposure), but as of 1995 public support in the US for GMOs remained as high as 73%.[15] Support in Europe was lower, but much higher than it would be by 1999 (see fig. 4). Most early victories (prior to 1996) for US anti-GMO activists did not involve widespread public outcry, but came in the form of pressuring specific links in food supply chains (particularly foodservice firms).[16] Partly as a result of nervousness or caution on the part of retailers and suppliers, products for direct human consumption were much more likely to be dropped than products intended for processing or animal consumption. Ron Herring writes that today “[i]ngredients such as soybean oil, corn starch, or corn syrup derived from the processing of GE feed crops are pervasively used by America’s processed and packaged food industries, but GE staple food crops, fruits, and vegetables intended for direct human consumption remain largely unplanted, even in the United States.”[17]
Through the mid-1990s, experts in biotech remained convinced that GM crops were poised for rapid uptake and adoption. Sociologist Rachel Schurman and political scientist William Munro, looking back over the adoption of GMOs in their 2010 book Fighting for the Future of Food: Activists versus Agribusiness in the Struggle over Biotechnology, argue that
[t]he scientific profession, the media, venture capital, and Wall Street were abuzz with possibilities these new ‘recombinant DNA’ technologies held out for generating a whole new industrial frontier and for solving a host of agriculture- and health-related problems. For these enthusiasts, the new biotechnologies offered a novel way to shortcut the slow processes of traditional plant and animal breeding, raise agricultural productivity, and to make better and cheaper medicines, all while representing a potentially enormous source of profit for the firms involved.[18]
Schurman and Munro continue, writing that their “enthusiasm was infectious. Large corporations and finance… poured money into these new ventures and built a massive scientific-cum-business infrastructure dedicated to generating new discoveries and new products with recombinant DNA.”[19]
To clean meat advocates and researchers, this rings familiar.
By 1999, public opinions on GMOs in both Europe and the United States had soured. Nearly every EU country saw GMO opposition rise from 1996 to 1999, most by double digits.[20] France went from 46% opposed to 65%, Greece from 51% to 81%, Britain from 33% to 51%. For context, this is comparable to the rate at which support for same-sex marriage increased in US General Social Survey data from 2010 to 2014.[21]
Country | Opposed (1996) | Opposed (1999) | Change |
Austria | 69% | 70% | 1% |
Sweden | 58% | 59% | 1% |
Denmark | 57% | 65% | 8% |
Norway | 56% | 65% | 9% |
Greece | 51% | 81% | 30% |
France | 46% | 65% | 19% |
Germany | 44% | 51% | 7% |
Luxembourg | 44% | 70% | 26% |
Italy | 39% | 51% | 26% |
Britain | 33% | 53% | 20% |
Belgium | 28% | 53% | 25% |
Portugal | 28% | 45% | 17% |
Ireland | 27% | 44% | 17% |
Finland | 23% | 31% | 8% |
Netherlands | 22% | 25% | 3% |
Spain | 20% | 30% | 10% |
Fig. 4. Opposition to GM food in Eurobarometer surveys in 1996 and 1999. Adapted from Schurman and Munro, Fighting, 108 and Gaskell et al., “Biotechnology and the European public,” Nature Biotechnology 18 (2000): 935-38.
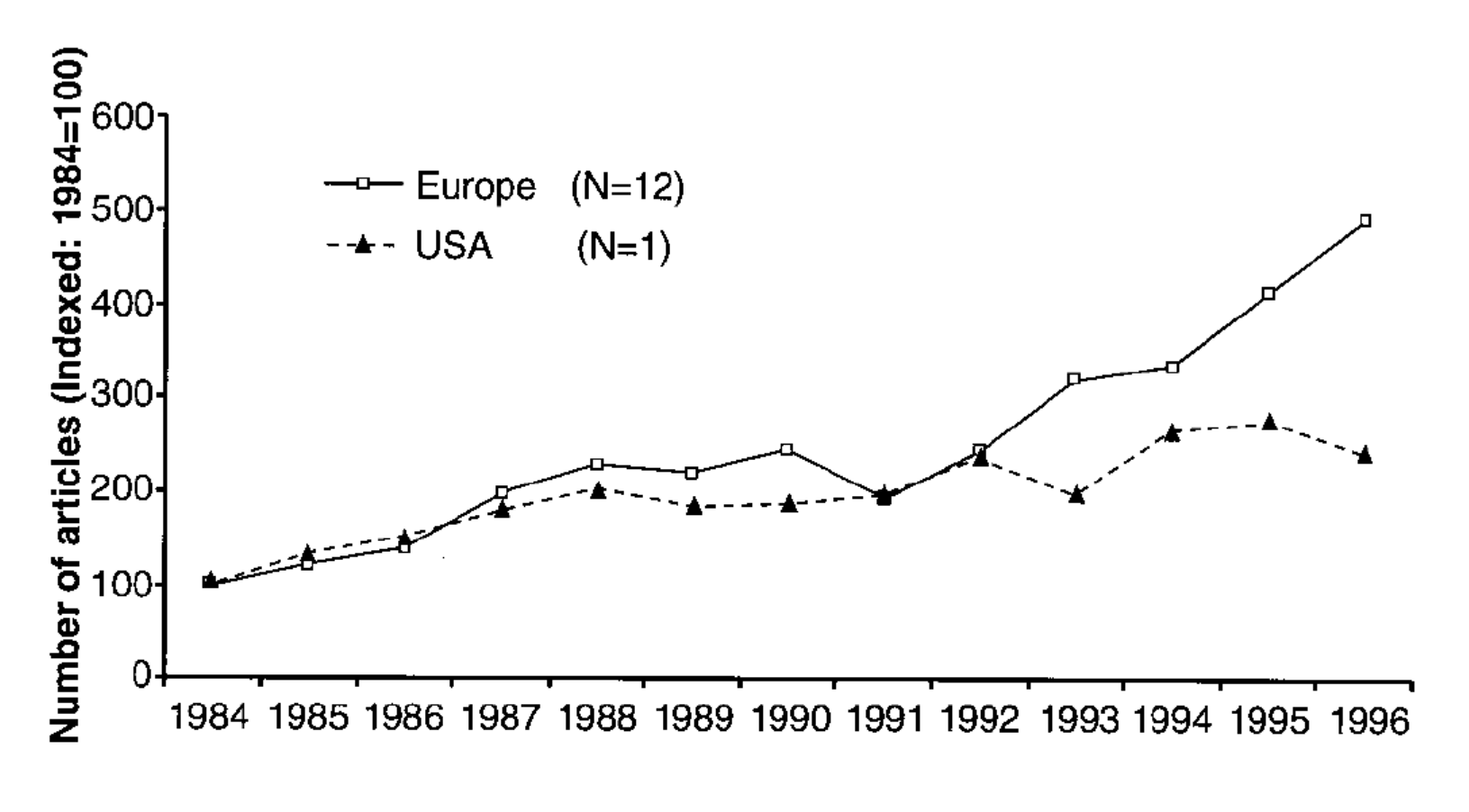
Gaskell shows that a greater increase in European press coverage of GM food from 1993 to 1996 preceded the greater rise in negative attitudes toward GM food among the European public.[22] Interestingly, he does not find a correlation between negative sentiment and negative coverage (indeed, European news sources were not reliably more negative than US sources in the time period studied), but between negative sentiment and coverage itself. Gaskell argues that this is consistent with the hypothesis that “in technological controversies it is the sheer quantity of press coverage that is decisive: The greater the coverage, the more negative the public perceptions.”[23] It is not clear, however, that this hypothesis would hold for different technologies in other contexts, such as a technology covered in overwhelmingly positive terms.[24]
Fig. 5. Number of articles on GM food appearing in twelve European newspapers and The Washington Post, 1984-1996. Gaskell, “Worlds Apart,” 386.
Public opinion polling in the US shows lower overall opposition than in Europe, but also a modest rise (between zero and eight percentage points) from 1995 to 2000, depending on which poll is considered. For example, the number of US consumers reporting that they would be less likely to purchase foods modified for insect resistance increased from 23% in 1997 to 27% in 1999. Those who said that such modifications would make them more likely to purchase modified foods declined modestly over the same time period, from 55% to 51% (although these numbers rose again in subsequent polls, reaching 58% in 2001 and 54% in 2002).[25]
In the late 1990s, GM crops were widely planted for the first time, raising their prominence not just as a hypothetical bugbear but as a concrete matter of public health. The late-1990s increase in GM planting was extremely rapid, especially in the US: global hectares planted with GM crops increased from 1.7 to 39.9 million hectares from 1996 to 1999, one of the fastest initial global adoption rates of a technology in history.
Year | Hectares (Million) |
1996 | 1.7 |
1997 | 11.0 |
1998 | 27.8 |
1999 | 39.9 |
2000 | 44.2 |
2001 | 52.6 |
2002 | 58.7 |
2003 | 67.7 |
2004 | 81.0 |
2005 | 90.0 |
2006 | 102.0 |
2007 | 114.3 |
2008 | 125.0 |
2009 | 134.0 |
2010 | 148.0 |
2011 | 160.0 |
2012 | 170.3 |
2013 | 175.2 |
2014 | 181.5 |
2015 | 179.7 |
2016 | 185.1 |
Total | 2,149.7 |
Fig. 6. Worldwide hectarage planted with GM crops, 1996-2016. Biotech Crop Highlights in 2016, International Service for the Acquisition of of Agri-biotech Applications, accessed March 16, 2018, http://www.isaaa.org/resources/publications/pocketk/16/.
In the late 2010s, GMOs are somewhat widely grown, especially in the United States, Brazil, and Argentina. However, they are not as widely planted or consumed as most experts in the 1990s thought they would be.[26] Only one GM crop, a strain of Bt corn, can be legally cultivated in Europe. Spanish farmers grow it in modest quantities (in the 100,000-hectare range). In general, the EU has remained quite closed to GMO deployment. The furore over GM food in Europe began to negatively influence public perceptions in the rest of the world. Poor countries dependent on agricultural exports were especially sensitive to the idea that they might lose access to European markets if their crops were seen as “contaminated” by genetic engineering. Other countries, poor and rich alike, took from European controversy nonspecific reasons to fear GM food: if genetic engineering was considered unsafe “by the Europeans, something must be the matter with it,” and so “even though there was no incontrovertible proof of any negative health effects caused by the technology, the idea that the scientific jury was still out and that serious problems could present themselves in the future traveled rapidly around the world, riding on currents of press coverage and the Internet.”[27] Anti-GMO activists’ victories in Europe have reverberated in other markets. Schurman and Munro note that
the box of potential solutions to the challenges of agricultural productivity and sustainable development in the twenty-first century looks far more open than it did ten years ago. The criteria on which these solutions are to be judged have expanded significantly. And the range of voices debating them has become much wider. The course of this technology has been altered significantly, and its future, once so clearly envisioned by its proponents, is far less assured.[28]
Today, GMO adoption continues to grow incrementally, although most gains come from areas where GM crops are already widely planted. Laboratory work to develop new GM products continues, albeit more slowly and with fewer funds than if the market for GM crops were larger. Thomas Bernauer argues that the most substantial obstacles confronting GM food adoption today are “low consumer trust in the safety of the food supply in key markets” (especially in the EU), concerns about “long-term health and environmental effects,” questions about corporate control of food supplies, and “insufficient consumer benefits from GE products.”[29] Messing with genes seems risky, large corporations are involved, and GM food doesn’t seem any tastier or safer, in part because most agricultural GM applications have gone toward fractional cost decreases and yield increases, both of which are less apparent to consumers.
Activists played a complicated role in bending the adoption curve for GM food. The next section further explores differing GM adoption in Europe and the United States and how activists influenced outcomes in both markets.
Differing adoption in the United States and Europe
What were anti-GMO activists able to accomplish in the US, Europe, and elsewhere? Ron Herring argues that the anti-GMO movement’s most important success “has been to construct a risk narrative of threatened common interests (e.g., safety, environment), based on a discourse of corporate dominance and exploitation, leading to empowerment of regulators with precautionary logic.”[30]
Why did anyone interpret recombinant DNA technology as a problem when there hadn’t been any public health disasters associated with genetic engineering and most early experts were optimistic? Early critics, Schurman and Munro tell us, “devoted themselves to developing a collective analysis of the technology” that included educating the public about what they saw as perils of genetic engineering with the aim of “push[ing] government policies in more precautionary direction.”[31] The early years of anti-GMO activism were often academic in nature: “From… the 1980s, leading anti-biotech activists interacted and consulted intercontinentally from their home bases around the world. They organized international conferences, shared ideas and information, and supported one another’s efforts. The intellectual architecture of the anti-biotech movement was constructed in these interactions.”[32]
These critics “transformed” advances in biotechnology “from an elite technological development into a highly contentious social problem.” They insisted on moving genetic engineering and its products from “scientific labs, corporate boardrooms, and government offices” to being “widely debated within different societies and among different segments of those societies.”[33] Activists challenged “science and profitability” as the primary criteria of evaluation and “injected an entirely different set of values into the discussion.” Those alternative values—loosely centered on ideas about the primacy of community, self-determination, purity, health, anti-corporatism, the sanctity of both nature and consumer choice[34]—have shaped contemporary discussions around GMOs to such a degree it is difficult to imagine these conversations without them.
Europe
Unlike the US, which grows genetically-modified crops in large quantities in the form of soybean, corn, cotton, canola, squash, papaya, alfalfa, and sugar beet, Europe grows one crop, Bt corn, in modest quantities in one country. As recently as the 1980s, the EU had no significant rules regarding GM crops. In 1999, the country imposed a moratorium on GM approvals.[35] The moratorium was lifted in 2004, but remains in practical effect. Only one additional GM species, the Amflora potato, won approval in 2010, but was pulled from the market and had its approval annulled by the General Court of the EU in 2013.[36]
Why does Europe grow so much less GM food than the US? Schurman and Munro describe a two-pronged victory for anti-GMO activists in Europe: (i) pressure on food retailers led to clearing shelves of products containing GM ingredients while (ii) a “political shift at the level of the European Union” made possible the 1998 moratorium which has remained essentially intact for twenty years.[37]
Pressure on food retailers
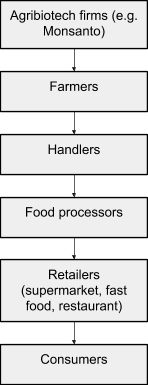
Activist pressure on food retailers led to a somewhat abrupt victory when Iceland Foods, a supermarket chain based in the UK, announced it would stop using GM ingredients in its own-brand goods. Iceland’s announcement set off a cascade of similar announcements by other European retailers. The companies renouncing GMOs included “virtually every major supermarket chain and food manufacturer on the continent as well as the British Isles.”[38] Four main factors drove this sudden turn of events. To explain these factors, it’s necessary to map the structure of food commodity supply chains, which, generalized and simplified, look a bit like this:
Fig. 7. Agricultural commodity supply chain. Arrows mean “sell to.” Created from description in Schurman and Munro, Fighting, 109.
Because each link in the food commodity chain is dependent on the buyers beneath it, pressure on one link in the chain tends to move upward (against the arrows). Activist pressure from one source, moreover, can intersect with and intensify pressure at other levels as it moves up the supply chain. For example, consumer pressure from beneath moved up the supply chain when supermarkets like Iceland Foods renounced GM ingredients. At the same time, food retailers and processors were growing increasingly skeptical of US agritech firms as reliable partners offering lucrative product innovations.[39] These forces intersected midway up the supply chain and rose further. For example, a variety of farmers who weren't even in the EU refrained from planting GM crops because European food handlers, processors, retailers, and customers might refuse to purchase them. Once farmers were refusing to accept GM seeds, agritech firms themselves were forced to cut back their development and marketing of GM products. This climbing of the supply chain played a crucial role in GMO defeats everywhere it occurred, but was especially salient in Europe.
Second, European supermarkets were unusually susceptible to activist pressure because twenty years of mergers and concentration in the sector (from the 1980s through the 1990s) created an environment where firms were few and powerful but extremely competitive with one another, often on the basis of perceived food quality. While the United States had relatively high levels of firm concentration among handlers and processors relative to retailers, European retailers were more concentrated than were European producers and handlers, leading to a situation in which retailers in Europe were more susceptible to activist pressure and more able to make their concerns travel up the supply chain than were US retailers.[40] “Supermarkets,” Schurman and Munro write, “occupied a position in the commodity chain that was both very powerful and very vulnerable.”[41] Therefore, they were both likely to succumb to perceived consumer pressure and possessed the ability to effect sweeping changes in ingredient lists and food processing. This combination likely led to a quicker and more a dramatic rejection of GM ingredients than would have otherwise occurred.
Third, European consumers grew more concerned about food safety, more concerned about food quality, and less trusting of government attempts to ensure either of these. This process was marked by a gradual shift in consumer thinking punctuated by occasional crises that stoked consumer fears, like the March 1996 announcement of bovine spongiform encephalopathy (BSE, also known as mad cow disease) outbreaks in the UK.[42]
Fourth, as mentioned, European food retailers and processors came to view US biotechnology firms like Monsanto as arrogant liabilities rather than valuable partners. This view could have been overcome, however, if it weren’t for the asymmetric relationship between agritech firms, who needed to sell to processors and retailers, and processors and retailers, who did not need to sell to agritech firms. European food processors already had non-GMO sellers available to them, further reducing any dependence on US firms that might have otherwise existed. This meant that European businesses further down the supply chain had a great deal of leverage to turn down GM products. Agritech companies did themselves few favors, either, when they failed to engage with European buyers to assuage their concerns and sell genetic engineering technology to them.
At the same time that these factors were contributing to a cascading renouncement of GM products from European store shelves, a parallel shift was taking place in Europe’s centers of political power.
Political shifts leading to moratorium
The political campaign that would lead to the EU moratorium on GM crops achieved its first major victory in a 1995 decision by the European Parliament around biotechnology patenting. Since at least 1988, the EU had been considering legislative action to bring its patent laws around biotechnology in harmony with standards in the US and Japan. The Patent Directive law meant to accomplish this was favored by biotech companies and opposed by activists, who
saw the directive as part of an alarming new trend toward life patenting that had started with the U.S. Supreme Court’s decision in Diamond v. Chakrabarty and was spreading to Europe. In their view, the directive represented a major expansion of European patent law to cover a much broader array of living organisms… biotechnological processes, and information. It would make genes into the “currency of the future” and give industry control of the whole supply chain, from basic genetic material to the products that make use of those genes and genes sequences, as well as future generations that carried that genetic information.[43]
In 1995, the European Parliament voted 240 to 188 to reject the patent directive. Activists had successfully pushed Parliament members to consider “ethical issues, including the philosophical and moral implications of patenting (and thus claiming private property rights over) human genetic material and medical treatments.”[44] The vote was a surprising victory for anti-GMO activists, but it wouldn’t last.
In 1997, the European Parliament passed a modified version of the directive that added “strong language that guarded against the patenting of human gene therapies and provided some limited protection to farmers engaged in seed saving.”[45] Even in its modified form, the passage of the patent directive was understood by all sides as a victory for biotech companies over anti-GMO activists. The initial 1995 vote against the directive, however, presaged the coming EU moratorium on GM crops.
The road to the EU moratorium began with a successful activist strategy to use sympathetic countries to slow or stop the approval process via scientific risk information requests. First, activists identified EU member states with anti-GMO sympathies. “Austria, Denmark, Greece, and Luxembourg,” for instance, “had all taken very cautious attitudes toward GMOs in their own countries and were motivated to create a strong set of biotechnology regulations at the EU level…. Accordingly, these countries were quite willing to object to an application on risk-based grounds or to request additional data, or both, before agreeing to render their decision” on a GM product petition.[46] Activist “groups such as Greenpeace and Friends of the Earth–Europe worked hard to support these countries’ competent authorities and to slow down the approval process for GMOs. They facilitated the access of” competent authorities to scientific papers and scientists who provided evidence that a given GM product was riskier than biotechnology firms maintained.[47]
According to activists, biotechnology firms themselves made several missteps during this process that the activists were happy to exploit. These included overconfidence, a lack of preparation, and little appreciation for the difficulties they would face. One activist interviewed by Schurman and Munro said that the
biotech industry was not too clever. The things they were applying for permission for were not particularly well-developed, not very well thought-out. Their applications were really quite sketchy, so it was easy to critique them…. The activists were more ahead of the game than the biotech companies were. [The companies] just thought it was going to be OK…. They’ll just send the papers in, and you know, nothing to worry about. So they were a bit taken aback when they started to get a lot of flack.
In 1998, three things happened together. First, the volume of scientific risk requests caused the EU’s GMO approval system to judder to a halt.[48] Second, European public opinion was in the midst of its 20-point swing against GMOs discussed earlier. Third, several major European governments became increasingly unwilling or unable to ignore public opinion on GMOs. The governments of France and Britain, which had supported biotech development in the past, reversed themselves in consequential ways at crucial moments. The French, purportedly biotechnology supporters as recently as 1997, began to waver in the teeth of protest at home before converting completely, pushing in 1999 for “a suspension of all commercial GMO authorizations” by the EU.[49] The British retreated from their pro-biotech positions “as [the British] public protested the democratic deficit in policymaking and called for greater transparency and public accountability on food issues.”[50] These conversions by Britain and France, as well as growing anti-GMO sentiment in virtually every EU member state, paved the way for the de facto moratorium on GM crops still in place today.
These and other activist victories also came about by shifting the frame of evaluation for GM goods from a product-based system to a process-based one. A process-based framework allows a GM/non-GM distinction to serve as the starting place for the evaluation of every new product seeking approval in the EU. This framework, codified by the EU in 1990, meant that all products developed with genetic engineering were automatically subject to more substantial regulatory barriers. Instead of using a product-focused system (as does the United States) where products are generally tested on the basis of their end-of-supply-chain safety, actual production processes would themselves constitute grounds for suspicion. This allowed GM products, which are as a technical matter quite different from one another and use a variety of loosely-related techniques to accomplish unrelated goals (e.g., developing cotton resistant to a species of weevil and a tomato that ripens slowly are different endeavors) to be grouped together both legally and cognitively under the same umbrella of suspicion.[51]
One of the key steps in the European GMO struggle was the work of activists to develop “an alternative discourse on biotechnology challenging ‘expert knowledge’ and trying to influence the construction of regulatory frameworks at the level of national governments and the EU as a whole.”[52] In existing technical discourse, for example, there was little reason to think that the act of using genetic engineering was itself dangerous and so no cause to institute a process-based system. There was little reason to think that GM lab techniques were more dangerous than traditional breeding techniques as a way of changing an organism’s genetic makeup. However, in anti-GM discourse, the presence of non-traditional gene alteration was itself grounds for suspicion. By developing and advancing an alternative technical and cultural discourse on what a GMO was and why it ought to be scrutinized, the “anti-biotech activists brought their worldview into the political sphere at” the levels of policy and public opinion.[53]
Europe compared to the United States
Unlike Europe, the United States plants and consumes GM crops widely. As mentioned, the US plants 39% of the world’s GM food, far higher than any other country. Despite ranking third in global food production (behind China and India) and even lower in hectares planted, the US is a runaway first in GM planting. By 2006, “almost 90 percent of the U.S. soy crop, 83 percent of the cotton crop, and 60 percent of the corn crop were genetically engineered, and thanks to the ubiquity of GM corn syrup, corn oil, and canola oil in processed food, GMOs had come to form part of almost every American’s daily diet.”[54] Unlike Europe, the United States never enacted a moratorium (de jure or de facto) on GM crops and did not erect significant procedural roadblocks to GM products. US attitudes toward biotechnology remain more positive than in those in Europe.
The scholarly literature suggests the following explanations for the differences in adoption between the United States and Europe:[55]
- “[F]arm lobbies” in the US “were more prone to defend GM crops” than in Europe “because the first GM crops—soybeans and corn—were far more widely grown [in the US] compared to Europe.”
- Europe’s agricultural chemical industry had a “larger presence” and wielded “greater influence” in Europe relative to the US and was also “threatened by GE crop innovations that would require less chemical use.”
- European governments may have exhibited “greater responsiveness… to consumer attitudes and expectations” than did federal and state governments in the United States.
- “Differences between political cultures in the United States and Europe” also contributed, e.g. the US tended toward self-described “risk assessment based on sound science” while the EU preferred “precautionary policy.”[56]
- The United States tends to use litigation (e.g., class action lawsuits) as a form of post-hoc regulation while in Europe “litigation is not as easily undertaken or as widely practiced as in the United States” and therefore “public safety is more likely to be guarded by regulatory systems set in place before the fact.”
- Europe has robust anti-GMO Green parties and political systems that allow those parties to participate in government even where they have not claimed electoral majorities or pluralities. Europe’s “multiparty political systems have given Green Party candidates—who oppose GM crops—a larger opportunity to gain seats in national parliaments or even control environment ministries inside national governing coalitions,” leading to “more veto points and veto players.”[57] The United States has a smaller, weaker Green Party and fewer avenues for minor parties to access power in general.
While not always set forth in the existing literature, the following differences were likely important:
- Early approaches to biotech regulation in the US came at the height of the Reagan years. When biotech firms like Monsanto proactively went to the federal government to build a regulatory framework they were met with regulators reluctant to do so: Monsanto executive Will Carpenter “ended up arguing not just with… biotech companies… [that] opposed any special regulations for biotechnology, but also with government officials themselves. True-blue Reaganites and even some career civil servants took the view that a drug produced using genetically altered bacteria should be regulated just like any other drug.” Decades later, Monsanto executives blamed this recalcitrance for souring the public on GM products. Monsanto's head of regulatory affairs, Leonard Guarraia, argued that anti-regulation FDA spokesman Henry Miller “did more harm to biotechnology than [anti-GMO activist] Jeremy Rifkin ever did. He put the government completely at odds with the critics.” Will Carpenter adds that Miller “thought he was helping us. But I told him that we couldn’t stand much more of his help.”[58] Rifkin himself, quoted in the New York Times, said that “If the F.D.A. had required tests and labels, ‘it would have been more difficult for us to mobilize the opposition.’”[59]
- The US regulatory system is more centralized than the EU system, where “more institutional access due to multilevel and decentralized policy-making… enabled agri-biotech adverse interests in Europe to exert more influence on agri-biotech policy-making. In the [US], low public outrage and a centralized regulatory system for agri-biotechnology… acted against agri-biotech adverse interests.”[60]
- Additionally, a process of ratcheting up to stricter regulation (rather than “downward harmonization”) took place between European states. Several European governments raced to demonstrate that they took GM concerns more seriously than their neighbors. This dynamic was weaker in US in part because US states have fewer options to regulate biotechnology themselves.[61]
- Consumer culture differs in the United States and Europe. European consumers showed more concerns about food quality and the details of food production than did US consumers, who “showed more concern about the convenience and price of food than they did about whether or not it was genetically modified.… Hence, one of the tactics that had worked most effectively for European activists turned out to have no purchase in the U.S. context.”[62] (Comparatively little purchase, I would say, rather than no purchase at all, but the point stands.)
- Public attitudes toward those regulating and deploying biotechnology tend to predict eventual support or opposition for GM food. The US public feels more positively about government regulators like the FDA than do Europeans, who tend to trust environmental groups over regulators.[63]
- Higher concentration in the European retail sector created industry structures where the “collective action capacity of pro-agri-biotech producers” as well as their ability to resist activist pressure tended to be lower in Europe than in the United States. “In Europe, public outrage and NGO campaigns [drove] a wedge between biotech firms on the one hand and food processors, retailers, and farmers on the other hand,” reducing “the collective action capacity of pro-biotech interests.” Meanwhile, in the US, “a cohesive and well-organized pro-biotech producer coalition… prevailed due to lower public outrage and weaker campaigns by agri-biotech adverse NGOs. Differences in industrial structure (particularly, higher concentration, both in economic and organizational terms, of the retail sector in the European Union than in the United States) and associated rigidities” affected outcomes in each region.[64]
- The relative strength of “uncertainty avoidance” sentiment in different populations may influence receptions of technological change. Uncertainty avoidance differs quite strongly across countries: “Americans feel less need to avoid uncertainty compared to Europeans. The ‘uncertainty avoidance’ score for Americans is only 46, compared to 65 for Germans, 86 for the French, and 92 for the Japanese.”[65] (Japan plants no GM crops and requires labeling for most GM food imports.)
- GM foods lacked union support in key areas in France and other European countries. In the US, unions tended to be both politically weaker and more ambivalent on GM products.[66] “GMOs… lacked institutional support,” Marcel Kuntz writes, “from the main agricultural union (FNSEA) [in France] and its associated organisms, which are usually open to innovation. One of the reasons being that the ‘mad cow’ crisis was associated in the public perception to ‘modern’ agriculture and ‘unnatural’ practice.”[67]
It’s prudent to note that US activist efforts were not as effective as in Europe, but did shelve the GM potato, Roundup Ready wheat, and delay the use of recombinant bovine growth hormone.[68] Major US food producers (Frito Lay, Gerber, Heinz) switched from GM ingredients from fear of backlash in 1999.[69] In several cases, activist action raised expense-to-revenue ratios past affordability for biotech firms. The increased expense and uncertainty of bringing GM products to market dissuaded biotech companies from developing or introducing genetically-modified food.
Analogies
Early GM development began not with multinational corporations but with small biotech startups in the mid-1970s.
The research that would eventually produce GM organisms began not with hulking multinationals but, like clean meat, with modest labs at startups newly-hatched from academia.[70] Several of these startups had come directly out of academic labs, as in the case of Herbert Boyer’s Genentech. Virtually all of these startups were inspired by academic advances in early rDNA techniques, like the successful modification of E. coli by Herbert Boyer and Stanley Cohen in 1973 (described above). This sector grew rapidly, reaching just over 100 biotech startups by 1982.[71] The industry gradually underwent consolidation and a series of acquisitions. Eventually, a few large firms (like Monsanto) came to control the development and release of GM products. This has possibly affected the rollout of GM food negatively (see perceived corporate attitudes of secrecy and arrogance), although it is also possible that large companies offer the expertise and infrastructure necessary to scale products rapidly.
Clean meat technology has certainly passed into a phase characterized by rapid growth in the number of startups working to bring products to market. It is unclear if clean meat companies will undergo a similar phase of consolidation and absorption by larger firms, although clean meat investments by Tyson and Cargill reinforce the possibility.
Early expert attitudes around the technology were extremely optimistic.
When scientists “first developed the ability to cut and splice genes from one organism to another in the 1970s and 1980s, the prospects for this revolutionary technology looked,” to them, “remarkably open and bright.”[72] Much of the genetic engineering rhetoric around deploying technical solutions to problems like the ecological damage wrought by industrial agriculture presages later evaluations about the potential of clean meat.
Moreover, concerns about ecological and human wellbeing motivated many early developers of genetically-engineered products, even at places like Monsanto.[73] The early days of genetic engineering (from the mid-1970s to the early 1980s) are marked by predictions from those working on the technology that world-changing innovations would be delivered within five to ten years.
A large amount of present-day clean meat discussion and evaluation resembles expert opinion in the early days of genetic engineering.
Patents and control of intellectual property mattered from early stages.
“Patents were so normalized” in the biotech industry “that no one ever really stopped to think about them,” Schurman and Munro write.[74] Aggressive patenting, necessary or not, may have contributed to activist backlash and a souring of public opinion, particularly in Europe. “No Patents on Life” became one of the anti-GMO movement’s most visible and successful campaigns in the 1980s and 1990s. In Europe, the movement defeated an EU patent directive in an early blow against the biotech industry on the continent (although a modified version was passed two years later: see political shifts leading to a moratorium in Europe).
Because the production of clean meat involves specialized and novel techniques, intellectual property protection may play a central role. If intellectual property decisions within clean meat are framed as a “patents on life” issue or similar, this could contribute to controversy.
Appeals to nature and concerns about artificiality played a central role in early reception.
Sylvie Bonny offers a table, reproduced and lightly edited here, of reasons for GMO rejection by the public that cites several concerns about the environment and the idea of doing violence to nature.[75]
Type of risk | Fears and perceived risks |
Troublesome, violent gene transfer process | - transgenesis means transgression of the barrier between species - risk engendered by troubling the “order of the genome” - humans have insufficient knowledge of the genome to authorize such tinkering with the transfer of foreign genes (living organisms are not just “building blocks”) |
Health (for example Bt corn and glyphosate-tolerant soya) | - allergies, long term toxicity - insufficient safety tests raise fears of consumers as guinea pigs - gene coding for Bt toxin means consumers are eating continuously secreted insecticide toxins - gene coding for the enzyme which degrades glyphosate means that GMOs accumulate products of degradation |
Environmental | - gene flow towards related wild species can lead to “superweeds,” invasive plants, accelerated decrease in biodiversity |
Agro-economic | - gene flow towards nearby crops of the same species can lead to impure harvests, “contamination.” - problem of volunteer plants in the following crop (rapeseed) - risk of a drop in Bt or glyphosate efficiency, interesting molecules for use in other agricultural sectors |
Economic | - of little interest to consumers, product “imposed” by multinationals - increasingly dependent farmers who must buy seeds every year - difficulty for developing countries to access such technology (patents) - appropriation of genetic resources by a few large multinationals - GMOs symbolize privatisation of all resources, even genetic ones - “imperialist” technology because coexistence with non-transgenic production is difficult (gene flow) |
Agriculture and food production model | - reinforcing of the industrialized model, the limits of which have already been critically portrayed - consumer perception: they’re playing with our health to make more money (see BSE and contaminated blood) |
Sociopolitical motives (value systems and beliefs) | - innovation neither asked for nor desired, but set up solely for the profits of some multinational firms - no respect for consumer choice due to the presence of GMOs in many additives and fortuitous contamination of grain through gene flow - media showing scientists (or associates) opposed to GMOs - vacillation in the positions taken by public authorities - perception that “everything is messed with more and more” engenders a desire to return to nature - GMOs symbolize development towards a type of society which is viewed negatively - “Such progress, why bother?” (a certain loss of faith in science and progress) |
Fig. 8. Sylvie Bonny, “Motives put forward for GMO rejection: risks, fears and reasons for refusal,” lightly edited and adapted. See Bonny, “Why are most European opposed to GMOs? Factors explaining rejection in France and Europe,” Electronic Journal of Biotechnology 6, no. 1 (2003): 64.
Concerns about unnaturalness are visible in the prominence of a term like “Frankenfood.” The word invokes “the mad scientist… and his unnatural monster,” Nina Fedoroff writes, and was “first applied by a Boston College English professor in a letter to the New York Times in 1992.”[76] Sergio Dompe locates the genesis of naturalness concerns in the fact that “‘the words ‘genetic engineering’ and ‘biotechnology’... call up ‘a glaring contradiction between life and technology, the natural and the artificial, that generates concern and apprehension.’” Dompe considers the switch from the term “nuclear magnetic resonance” in hospitals to “magnetic resonance imaging.” “The moral of the story,” he writes, is that “[i]nappropriate words, such as a misunderstood adjective or a bold juxtaposition, often influence our view of reality, feeding our suspicions and unspoken fears even where there is no justification.”[77] There is little evidence that GM foods were ever able to leave behind naturalness concerns.
Clean meat has, of course, raised concerns around its naturalness or lack thereof. The term “Frankenmeat” circulates widely, and one doesn’t have to search long to find negative reactions founded in a suspicion of clean meat’s artificiality. However, the history of GM food indicates that concerns around unnaturalness alone are not sufficient to provoke widespread backlash (or else many medical procedures and drugs would go unused). The risk of backlash is highest, rather, when concerns from different areas overlap and intensify one another (e.g., corporate control of food meets unnaturalness).
The potential benefits of a new technology may positively affect its adoption, but the evidence is far from decisive.
It seems likely that technologies with large, obvious benefits are more likely to be adopted and less likely to face backlash. The evidence on this question, however, is surprisingly mixed. It is true that, as Bernauer writes, “Social science studies of risk show that consumers are more willing to accept risks if they perceive substantial benefits in consuming the respective good,” with cell phones, tobacco, and coffee as examples.[78] Often, however, tech with large benefits seems to be adopted more quickly on balance (e.g. Indian GM gray market seeds)[79] but does not face sufficiently reduced backlash risk. For example, Fedoroff relates that vaccines, when first introduced “against smallpox… were vilified in editorials and cartoons, publicly protested, and strongly resisted.” However, “national governments and the UN persisted in vaccinating people—sometimes even with a bit of coercion—and smallpox is gone.”[80]
Fig. 9. “Death the Vaccinator,” originally published by The London Society for the Abolition of Compulsory Vaccination, late 1800s. Preserved by The Historical Medical Library of The College of Physicians of Philadelphia, https://www.historyofvaccines.org/content/death-vaccinator.
The benefits clean meat may offer, if made clear to the public, may help accelerate its adoption and dampen potential backlash, but they are unlikely to function as a panacea and do not ensure its widespread uptake.
Framing remains paramount.
Throughout debates over GM food, successes and failures of different products would often turn on changes in framing and perception rather than shifts in underlying technological, economic, or agricultural realities. Christophe Bonneuil argues that as the framing of the debate over GMOs changed in Europe, “different heroes and victims were identified or constructed. For example, within the ‘ecological risk’ framing, the main victims were wild relatives of crops, and public-sector researchers carrying out biosafety research were heroic figures; but once the contamination of other crops became a key issue, the main victims were organic farmers and others choosing not to grow GM crops. The ‘right to information’ and ‘right to participation’ framings identified local politicians as failing to adequately serve and protect their constituencies.”[81] For example, an important shift in French discourse on GMOs in the late ’90s came about when “‘risk framing’ successfully challenged… ‘innovation framing’.”[82] A further example comes in Calgene’s and Zeneca’s marketing of their GM tomatoes and tomato paste as high quality because they had been genetically engineered, not in spite of it: Zeneca, for example, “spent an enormous amount of time cultivating British journalists and lining up partners in the food business. They’d already decided that this tomato paste would be packaged in special cans and labeled as the product of ‘genetically altered tomatoes,’ even though such labels weren’t required…. They even turned genetic engineering into a marketing gimmick, advertising the launch of tomato paste as ‘a world-first opportunity to taste the future.’” The experiment succeeded: “Through the summer of 1996 Zeneca’s red cans of tomato paste, proudly labeled ‘genetically altered,’ outsold all competitors.”[83]
Calgene and Zeneca’s examples reinforce the value of focusing on the positive aspects of a new product rather than endlessly rebutting fears and negative perceptions. This dynamic played a role in the adoption of nuclear power. Constant discussion of safety concerns, even if to answer them in a technically-sound manner, tends to replace positive frames of an issue with frames that center on whether a technology will cause cancer or annihilate endangered species wholesale—even if there is no evidence that these concerns are warranted.
Many of the framing shifts around GM food took place independently of any meaningful change in the underlying reality of the product or technology in question. The relevant industry actors, moreover, seemed unaware or unready for how quickly framing shifts could happen and how consequential they could be. Clean meat may be defined by “innovation framing,” or something like it, for the moment, but the history of GMOs shows how quickly such a frame can be overcome or punctured by a new, fear-motivated frame.
Non-experts often make decisions based on acceptability rather than risk.
George Gaskell et al. examine polling data from the US and Europe on GMOs and find that “[r]espondents with concerns about gene technology tended to think principally in terms of moral acceptability rather than risk—a significant difference from the way in which experts normally judge the acceptability of new technologies.”[84] A public motivated by moral acceptability is less likely to be swayed by arguments about the statistical safety of a new product like clean meat and more likely to be swayed by arguments that emphasize the product’s newness, uncertainty, and, therefore, potential unacceptability.
Disanalogies
GMO development required the planting of physical fields that became sites of controversy.
Fields of GM crops have to be planted to properly develop and test GM crops. This apparently mundane fact, as Bonneuil shows, played a nontrivial role in the French struggle over GMOs in the late 1990s. Researchers initially conceptualized test fields as deep within the research pipeline, not anywhere near consumers, and therefore not subject to public scrutiny. Activists and the public, however, came to see them as contestable because of their physical proximity to and location in the natural world. A “leader of the Confédération Paysanne,” an anti-GMO French group, argued that a field of “GM oilseed rape was only 500 meters from a non-GM seed production field” and that “the consequent risks of ‘genetic contamination,’” in part justified its destruction.[85] French activists in particular began to conceptualize GM fields as “‘an intrusion in the social space’” and the fields became places from which local opposition to GM crops grew, not unlike site-specific opposition to nuclear power plants.
Because clean meat production is unlikely to involve open-air planting or any scenario in which material from the production process may drift into surrounding areas, clean meat production is likely to be less susceptible to site-specific opposition than were GM crops.
Perceptions of corporate secrecy and arrogance
The high level of competition between early biotech firms, the desire to control key intellectual property, and the race to bring products to market may have led to increased levels of secrecy and aggressiveness within the industry. Observers of and participants in the early biotech industry describe a sense of urgency, even “adrenaline.”[86] As smaller biotech companies were absorbed, larger firms, Monsanto in particular, often maintained a hard-charging attitude toward preparing GM products for market. These firms came under pressure to make their large investments in biotechnology pay off in the form of lucrative new GM products. Genetic engineering projects were often chosen on the basis of potential market share and projected profits.[87] Moreover, shareholder value theory, ascendant in the 1980s, meant that executives were incentivized to generate short term profits rather than attend to environmental and social questions.
Monsanto, for example, refused to proceed slowly on introducing GM products to the European market. They “stormed” Europe, sending GM crops there unlabeled “despite being warned not to do so.”[88] This led to significant backlash on the continent.
While this has been discussed in the Europe section above, the details remain striking. Simon Best, director of biotech projects at Zeneca at the time, tried to caution Monsanto CEO Robert Shapiro about the company’s strategy in Europe, saying
“‘Look, you’re severely underestimating the food situation in Europe. If you don’t either label or start a communications program now, the food chain isn’t going to back you up. And there’s going to be a major consumer reaction. We haven’t had enough time yet to get over the labeling issue. If you just ship these things in as a surprise, it’s going to be a huge disaster.’”
….Shapiro was unperturbed. “We think you’re wrong,” he told Best. “Our people in Europe say that this is an exaggeration. We’ve talked to the right government people in all the countries of Europe”.... Best, for his part, thought Monsanto was behaving like a “uniquely arrogant company.” “At no point did they actually listen to the people who knew,... the food companies,” he says.[89]
In one sense, Shapiro wasn’t wrong: Monsanto had talked to the right government people in Europe, and by March 1996 had gained regulatory approval for Roundup Ready soybeans. The problem, also discussed in the section above, was that European customers remained less trusting of government regulators than US customers, so regulatory approval counted for little among the European public. British revelations of deaths from BSE (mad cow disease) just five days “after Europe voted to accept Roundup Ready soybeans” did little to boost public confidence in European regulators or food safety.[90]
Kurt Eichenwald, in a New York Times story from 2001, writes that Monsanto likely erred in introducing recombinant bovine growth hormone (rBGH) as its first product to farmers. Milk from rBGH cows became associated with direct human consumption, particularly by children, in a way that may have contributed to pushback.[91] Eichenwald further reports that
[biotech’s] go-slow approach was shelved [in the early 1990s] in favor of a strategy to erase regulatory barriers and shove past the naysayers. The switch invigorated the opponents of biotechnology and ultimately dismayed the industry's allies -- the farmers, agricultural universities and food companies.
“Somewhere along the line, Monsanto specifically and the industry in general lost the recipe of how we presented our story,” said Will Carpenter, the head of the company's biotechnology strategy group until 1991. “When you put together arrogance and incompetence, you've got an unbeatable combination. You can get blown up in any direction. And they were.”[92]
It is tempting for present-day clean meat companies to shake their heads ruefully at the bad old days of biotech. Many clean meat companies, it is true, act in a way that is on balance more transparent, open, and conscious of consumer reaction than the large biotech firms discussed here.
However, it is important to remember, first, that many biotech firms at the time genuinely believed they were working toward a knowledge whose dividends would be widely shared:
These young genetic engineers did believe that their work would be good for the planet, possibly making it easier to grow food or reducing agriculture's dependence on chemicals. Some of them, working inside chemical companies, often saw themselves as “green” revolutionaries fighting against the entrenched power of the chemists… They’d seen DDT banned and Earth Day celebrated. Chemicals represented a dirty and regrettable past, and biology was the savior.
At Monsanto those views “came from the very top,” says Pam Marrone, a researcher at Monsanto during the late 1980s. “I remember having lunch with [then-CEO] Dick Mahoney and him saying, ‘Because of parathion [a particularly hazardous insecticide], I don’t ever want to be in chemicals again. And that’s why we’re in biotechnology.’”
“During those years, all of us who went into biology were influenced by the wave of environmentalism,” says Willy de Greef, who worked… for Plant Genetic Systems [and] Novartis.[93]
Moreover, if interviews with researchers years later are to be believed, the working environment was far from toxic or cynical: “‘I had sworn I would never work in an industry,’ [Monsanto researcher Harry] Klee recalls. ‘But when I got to Monsanto, it was just instantly apparent that if I wanted to do plant biotechnology, this was the place to be.’ It wasn’t just that Monsanto offered superior resources, Klee says. Paradoxically, it was also a much more collegial place. In academia every colleague is also a competitor; every collaboration involves negotiation over credit. At Monsanto, Klee says, much of that was stripped away. ‘There was less ego involved.’”[94]
Many clean meat companies feel similarly about their work. The wider public may disagree, even when insiders are at or near apparent consensus. As Charles writes after interviewing scores of old genetic engineering researchers, their “self-image [of helping the world] held a hazard. Those who occupy, in their own minds, the moral high ground are usually the least able to accept criticism or even comprehend it. When the genetic engineers found themselves attacked by a new generation of environmentalists, they were incredulous and hostile.”[95]
Second, recall that genetic engineering was not always the province of large corporations. Paul Shapiro, differentiating GMOs from cellular agriculture products like clean meat, writes that “GMOs are largely… produced by megacorporations like Dow AgroSciences and Monsanto, in part to maximize the output of feed crops for animal agriculture. Synthetic biology for agricultural products, on the other hand, is primarily being used used by tiny start-ups seeking to solve key environmental problems by replacing traditional animal agriculture.”[96] This is certainly true. However, many of the early genetic engineering firms were exactly “tiny start-ups seeking to solve key environmental problems by replacing traditional… agriculture.” Even as these firms grew, they remained open and transparent. Calgene and Zeneca, for example, advertised their tomatoes and tomato paste as genetically engineered products (Calgene in the US, Zeneca in the UK). Calgene, as mentioned, even distributed a 1-800 number to field questions about genetic engineering. Aggressiveness and secrecy became public liabilities after older, larger firms came to dominate the production and distribution of GM products. (Calgene was acquired by Monsanto in 1997 and Zeneca merged with Swedish pharmaceutical company Astra AB in 1999.) Present clean meat companies may well be transparent, but can they guarantee they will remain so if absorbed into the larger food-supply system?
Firms like Tyson and Cargill are importantly disanalogous to Monsanto because they’re established food producers, not biotech firms. In many cases, clean meat seeks to replace the products of established food producers. This difference could mitigate potential backlash of the kind that afflicted Monsanto and could even prove advantageous for clean meat startups. However, much of the opposition to Monsanto was motivated by concerns and features of the company (e.g. size, pursuit of profit, implications around corporate control of food supply, attitudes of European consumer groups toward American corporations, patent concerns, strategic errors by executives) that are present or could become present in food producers. Either way, differences in industry type should make us more uncertain about whether uptake by all big companies is dangerous.
Unclear relationship
Early pioneering firms were absorbed by larger firms (or dissolved).
As mentioned, a profusion of over 100 small startups dominated the biotechnology scene in the 1970s and 1980s before being absorbed by larger, older firms like Dow Chemical and Monsanto. Indeed, most early biotech firms, even those that made technical contributions or were first to market with a novel product, did not grow into large or lasting companies. In a passage resonant for clean meat startups, Schurman and Munro suggest that
no matter how much brainpower and effort these scientists and their business counterparts poured into their jobs… small biotech companies faced an uphill battle in keeping their businesses alive. Conducting research using… new molecular techniques was intrinsically expensive…. While it was not difficult for a new company with a couple of distinguished scientists to interest some risk-oriented investors to support their endeavors for a couple of years, it was difficult to sustain that revenue stream…. What typically happened to firms… if they were lucky, was that a large corporation would say, “Well, you’ve really invented something, and we have money; we’ll help you finish.” They made people an offer they couldn’t refuse. For many start-up owners, being bought out by a bigger company or having one purchase a large equity share in the small firm was their best hope for staying in business.[97]
Mergers, acquisitions, and investments by larger firms can affect the trajectory of an industry by changing the incentives of employees and companies, business structures, the way firms secure funding, which endeavors are worth research and development dollars, the cost-benefit ratios of different products, market access, scaling costs, and so on. For example, executives at acquiring companies like Novartis and DuPont “came from industries that were heavily dependent on intellectual property protection… so the need to have property rights over scientific discoveries was a standard element of their business strategies… competing for patent rights over genes and gene transformations became a ‘first principle’ of the business.”[98]
Because industry-wide changes occur within complex systems, it is impossible to outline with precision the effects a given change in industry structure will have. Concentrating genetic engineering development in the hands of larger, older firms created liabilities in the form of negative public perceptions about the safety and acceptability of GM products. Clean meat development has not yet seen significant mergers and acquisitions, but it has seen investment from large firms like Cargill and Tyson. It’s not yet clear if clean meat will undergo a round of industry consolidation the way early genetic engineering did.
The evidence about a link between education and attitudes toward GMOs is mixed, and the connection may not be as strong as assumed.
Much of literature assumes or asserts that as consumers grow more educated (about biotechnology and in general), they become more supportive of GM products.[99]
Bernauer argues against a strong link between education and support for GM food:
There is no convincing empirical support for the assumption that people who know more about agricultural biotechnology are, as a consequence, more supportive of that technology. Consumer survey data shows that supporters of agri-biotech applications tend to perceive the technology as useful, morally acceptable, are less concerned about risks, and trust the safety of their food supply. Opponents hold opposite views. Some analyses… show that more engaged and informed [men]... with a higher education are slightly more supportive of the technology. But the causal relationships underlying such (statistically weak) correlations are… hard to fathom. One of the reasons… knowledge and agri-biotech support correlate only slightly might be that support for or opposition to the technology is driven by all sorts of motivations and values rather than the level of knowledge.
EU consumers, for example, who “appear better informed about agricultural biotechnology than US consumers… are not more ‘technophobic’ than US consumers, but are much less supportive of agri-biotech applications.”[100]
Education may be ineffective at converting consumers from one view to another in part because, as Ron Herring aruges,
despite widespread consensus on fundamental values – farmer welfare and sustainable agriculture – knowledge claims in networks built on trust and solidarity have reinforced a global cognitive rift on biotechnology. It is not normative dissensus, as in the historic contentions over abolition of slavery or female suffrage, but rather contention around knowledge claims integral to those normative positions. These knowledge claims in turn fit into receptors in rival networks contesting genetic engineering in agriculture along two global rifts.[101]
Herring’s “epistemic brokerage” thesis, if more true than not, contributes to a fatalistic view that contestation remains intractable as long as separate solidarity networks persist. It may also, however, highlight the importance of strategic moves within networks that adjudicate claims as true or false and the way structures of solidarity determine whether truth claims travel across cultural and educational frontiers.
A single dominant term emerged early.
Like “clean meat,” the term “GMOs” binds and seals together a disparate bundle of procedures and consumer goods. The singularity and visibility of the acronym GMO has probably had the effect of concentrating “anti-GMO” criticism on a group of loosely related products and techniques that are not, all things considered, all that similar to one another. This has also meant that the discussion around GMOs has been harder to disentangle and clarify than it would have been if it had discerned between different applications of genetic engineering. Herring suggests that a more productive discussion of GMOs would include more careful parsing of different “traits… cultivars… genetic events… conditions… developmental purposes.”[102] The binding effect of a dominant term is worth thinking about with respect to, for example, strategic consolidation around a term like “clean meat” and what might be compacted and sealed together by that term.
Concerns arose that GM food production would deepen existing centralization in the food system.
European activists in the 1990s “portrayed agricultural biotechnology as the latest trend in large-scale, industrial agriculture, one that carried the potential to destroy the thousands of small farms that dotted the European countryside. To many Europeans… this idea was deeply offensive. This discourse was particularly persuasive in France, where artisan agriculture and notion of ‘terroir’ [the characteristics of a crop that come from the environment in which it is grown] were part and parcel of people’s food identity and culture.”[103]
Clean meat production would, in all likelihood, swap one kind of industrial production (industrial slaughterhouses) for another (clean-meat plants that may resemble breweries or greenhouses).[104] Clean meat production could become relatively centralized or decentralized. Predictions of tabletop devices for consumers to print their own meat at home abound, but it is also the case that breweries (the production method possibly most similar to what scaled-up clean meat production will look like) tend to be large, centralized facilities demanding large capital expenditures and are consequently owned and operated mostly by large corporations.[105] However, current meat production is so centralized that it would be quite hard for clean meat to increase overall centralization. For this reason, slight decentralization seems more likely, although the effect is unlikely to be substantial in either direction.
Cultural mismatches between companies and the markets they were selling to affected attitudes toward GM products.
Europeans saw Monsanto as an “Ugly American” company, and GM food adoption suffered as a result.[106] For the European public (and some European food companies and regulators), the company’s actions were marked by “arrogance, cultural insensitivity, and a deeply held belief that ‘our way is better.’ In a manifestation of [its] corporate culture… Monsanto stormed into Europe like a general going to war, making one cultural and political gaffe after another in its dealings with the European public and governments.” (See, obviously, perceived attitudes of secrecy and arrogance.) In addition to the shipping of unlabeled GM soy to Europe (the action that Zeneca’s Simon Best had warned Robert Shapiro against), Monsanto engaged in a tone-deaf advertising campaign in the UK in which it made claims, seen as overblown and unsubstantiated, to the effect that GM crops would make possible “a tomorrow without hunger.” “Collectively,” these “miscalculations made Monsanto into the perfect target for activists, enabling them to vilify the firm and the technology simultaneously.”[107]
The furor in Europe poisoned Monsanto and genetic engineering’s reputation beyond Europe. Outside of Europe and North America, “it is extremely difficult for politically cautious leaders in poor countries to be seen welcoming GM seeds if they are coming from a private corporate lab in the United States.” A variety of governments evince an anti-corporate skepticism toward GM crops: “One reason,” pro-GMO political scientist Robert Paarlberg testified before the US Congress in 2001, that “Kenya has not yet given final biosafety approval to the virus-resistant sweet potato is that the technology came originally from the Monsanto Company. One reason it has been hard in Brazil to get approval for RR Soybeans is that… this is a Monsanto product. One reason India has not yet given a final release to Bt cotton is that it is… a Monsanto product.”[108]
It is possible that smaller startups with more flexible and transparent company cultures are less susceptible to the dynamic that ensnared Monsanto. Even if they are, would that difference fade if clean meat underwent the consolidation and scaling that happened among genetic engineering firms?
Safety incidents, even if unrelated to technology in question, can influence public opinion negatively.
As mentioned, the 1996 mad cow scare in the UK “undermined consumer trust in expert opinion after... public health officials gave consumers what proved to be a false assurance that there was no danger in eating beef from diseased animals.”[109] Even though there was no connection between GM food and BSE, mistrust of regulators and GM food worsened and anti-GMO activists, including Greenpeace, took advantage of crisis.[110]
Bernauer summarizes the conventional wisdom that incidents arousing public concern about the safety of food and biotechnology tend to reinforce one another:
it is widely assumed that the BSE crisis and other public health and safety scandals (e.g., the dioxin scandal in Belgium in 1999 and HIV-contaminated blood in France and elsewhere) in the second half of the 1990s… dealt another blow to public trust in regulatory authorities and the scientific expertise on which they rely. These crises have also increased the receptiveness of the media to public health and environmental issues. Thus, they have contributed indirectly to more negative press coverage of agricultural biotechnology.[111]
Marcel Kuntz adds that “[o]n November 1, 1996, the French leftist newspaper Liberation launched the media” condemnation “of GMOs by its front page headline ‘Beware of mad soya (Alerte au soja fou)’. The crisis took short the [French] government which was rather supportive of agricultural biotechnology.”[112]
It is certainly plausible that a public health scare could negatively affect perceptions of clean meat. It is also plausible that such a scare, especially if localized to meat products, could raise public fears of eating slaughtered meat and increase relative demand for products than can demonstrate their safety, like clean meat.
Opposition to GM research and products arose as part of a broader radicalization.
Recall that appeals to nature and concerns about artificiality played a central role in the reception of GM products. These concerns were part of a broader political and cultural movement in the second half of the 20th century through today of increasing suspicion and resistance toward governments, corporations, processes of globalization, modernity, and scientism.[113] Links between different activist causes play an important role in the history of anti-GMO activism. When “protestors shut down a meeting of the World Trade Organization in Seattle in December 1999, opponents of genetic engineering took their place beside marching steelworkers, religious activists demanding cancellation of poor countries’ debt, and defenders of tropical forests.”[114] Apparently unrelated causes can be found in close proximity to one another, mutually sharing resources, knowledge, and awareness.
Much of the concern about genetic engineering arose as part of a broader radicalization. For example, Jeremy Rifkin, who would later become famous as an anti-GMO activist, “had been radicalized during the Vietnam War years. Rifk[i]n had grown up in a politically Democratic but socially conservative working-class community on the South Side of Chicago” and was an undergrad the University of Pennsylvania when the Vietnam War started, “setting him on a new path.” He had “started writing for a small, left-leaning magazine” when he “learned that some pharmaceutical companies were working with rDNA technologies.” Rifkin then cowrote Who Should Play God?, a 1977 anti-genetic engineering book that sold well and influenced the early anti-GMO movement.[115]
Links and associations among activists groups and in how an object of political attention comes to be linked with other causes mattered a great deal for GM foods and will matter for clean meat. It is possible that a close link between clean meat and veganism and environmentalism may help avoid certain types of backlash faced by GM foods. But clean meat could be subject to other associations that are worth being on guard about, like unnaturalness or the similarities to GM foods.
Increased public awareness was linked with increased negative sentiment.
The double-digit drops in support for GM products in Europe documented earlier coincide with activist efforts that, starting in 1996, led to “20 point increases in awareness” of GM food in multiple European countries.[116] This is not sufficient in itself to prove that increased awareness causes negative sentiment (e.g., it is possible that a positive campaign around a technology could increase awareness while increasing positive sentiment), but, along with some additional evidence,[117] it is suggestive.
Rae Goodell documents a very early case, perhaps the first anywhere, in which the Cambridge, MA, city government had to decide whether to regulate a recombinant DNA facility operated by Harvard University. Goodell characterizes rDNA policy in those years (1973-1975) in Cambridge as dominated by scientists and researchers who were not sensitive to or interested in public opinion.[118] Three groups then arrived on the scene in successive waves: press, other professional academics and intellectuals, and Congress. Because of these arrivals, “in Spring 1976, a remarkable change occurred: the city government of Cambridge, Massachusetts[,] tackled the issue, and a group of local citizens began to involve themselves in the DNA regulatory process.” Goodell finds that, “[a]ccording to subsequent interviews, the Cambridge City Councillors quickly gave up trying to understand the technical issues. Instead, they focused on one basic decision: was the DNA issue important enough to warrant city action?” Notability, rather than any technical safety issue, became the main criterion for regulation. The councillors found that “the DNA issue” was important enough to “warrant city action,” on the basis of “three essentially non-technical grounds: (1) the size of the response from Harvard and MIT, the press, and the scientists; (2) the size of the rift between proponents and opponents, with impressive credentials and heavy emotion on both sides; and (3) their view that the scientists at Asilomar, NIH, and Harvard had been delinquent in their provisions for public involvement.” The fact that rDNA research seemed important became evidence of its importance and the fact that it seemed controversial became evidence of its controversiality.
It’s hard to say if increased awareness of clean meat will lead to increased negative sentiment, in part because public discussion of clean meat has been more positive and less focused on public health risks than was discussion of genetic engineering.
Supply chain structure influenced the behavior of distributors and retailers.
As described above, the wave of supermarkets dropping GM ingredients in the late 1990s was made possible by highly competitive retail firms who couldn’t risk losing customers, by a supply chain structure in which sellers were susceptible to pressure from buyers, and by the failure of American biotechnology firms to secure buy-in from European processors, handlers, and retailers.
Supply chain dynamics are likely to matter as clean meat is brought to market. Securing buy-in from retailers and other distributors has already been a matter of consequence for plant-based meat companies like Impossible Foods (whose partnership with the largest food distributor in the United States, Dot Foods, was crucial to its expansion) and Beyond Meat (whose products Whole Foods began carrying nationwide in April 2018). It is too early to tell if Impossible and Beyond products will resemble Calgene’s Flavr Savr, a novel product from a young company that sold well and generated interest before being discontinued, or will become permanent, scalable components of the food supply.
Consumers who live in places with mandatory labeling are “more critical of the technology.”
Whether because of correlation, causation, or (most likely) a combination of the two, consumers “in countries with mandatory positive labeling [for GM food] are usually also more critical of the technology.”[119]
Missouri state house bill 2607 (2018)[120] and the US Cattlemen’s Association petition to the USDA[121] attempt to impose de facto labeling requirements on clean meat. It is possible that clean meat manufacturers will (following Zeneca and Calgene thirty years before them) proactively label their products as different from slaughtered meat and drive interest that way, although prohibiting the use of the word “meat” could engender customer confusion. Labeling is likely to become an increasingly contentious issue in the clean meat sector.
It may have been the case that GMO producers were too slow to organize a group for themselves in the EU.
“Most analysts,” Bernauer writes, “claim that the unwillingness or inability of input suppliers to organize in time is responsible for the industry’s inability to prevent the European Union’s 1990 decision to focus on process-oriented agri-biotech regulation.”[122]
It is unclear how clean meat will be regulated, but already a petition by the US Cattlemen’s Association to the USDA to disqualify the term “meat” from being used to refer to non-slaughtered meat indicates that the clean meat industry is likely to find utility in some kind of industry advocacy group. (Pushback to the Cattlemen’s Association petition has taken the form of a comment sent to the USDA by the Good Food Institute and signed by seven other plant-based food companies, in addition to lawyers for or CEOs of clean meat companies giving quotes to reporters about labeling and the USDA.)[123]
Support for medical biotechnology consistently outpaces support for food biotechnology.
Interestingly, support for medical biotechnology remains high (in the 57-91% range in the US and EU) while support for food biotechnology is on average 30 points lower. Additionally, Gaskell shows that as opposition to GM food rose ~25 points from 1996 to 1999, opposition to medicine rose about 2.5 points.[124] Some small correlation can be observed, but it is quite limited.
Herring argues that the reason for the split in opinions on medical and agricultural biotech
cannot be poor public understanding of recombinant DNA (rDNA) science, as the science of recombinant drugs is just as mysterious to ordinary citizens as that of GE crops. Nor can corporate control be the dominant issue, as the market for recombinant medical drugs has been just as corporate-led as the market for GM seeds. Similarly, high product costs cannot be the issue, because recombinant drugs are of course far more expensive than GM seeds. Nor can the greater dependability of regulations explain strong support for recombinant drugs versus GE crops, because drug regulators have made tragic errors over the years.[125]
Herring presents reasons to revise downward the weights we might give to these explanations, but I would not discount them in the stark terms he does here. To replace these explanations, Herring nominates the fact that GM foods have a higher chance “of involuntary exposure, and also [of] environmental release. Risk theorists know that public acceptance becomes less likely when personal exposure to a suspected risk is perceived as involuntary.”[126] GM crops are planted in fields and grow outdoors. Generally, GM medicines are not and do not. At a higher level, it seems likely that the different assumptions and perceptions attached to medicine and food play a role. Biomedical research is not foreign to medicine, it is medicine, and offers success after success as proof it belongs. Agricultural biotechnology, on the other hand, invades otherwise “natural” processes of cultivating land and growing food, and the genetic engineering of food offers no well-known successes in the way of polio vaccines and penicillin.[127]
Mid- and later-stage considerations
As GM products and the debate around them have spread and aged, complex international phenomena like regulatory differences and trade have deepened and complicated existing barriers to adoption. Bernauer argues, for example, that “global regulatory polarization and trade conflicts have exacerbated already existing domestic controversies over agricultural biotechnology.”[128] (“Global regulatory polarization” just describes the fact that the EU regulates agricultural biotechnology strictly, the US in a more relaxed manner, and the rest of the world finds various balance points.) Moreover, Bernauer argues, “prevailing public and private sector policies do not add up to an effective strategy for mitigating or overcoming regulatory polarization, diffusing trade tensions, and creating a long-term global market for [agricultural genetic engineering] technology.” These nonoptimal public policies include
establishing ever more complex and stringent regulations that are increasingly divorced from scientific evidence and insufficiently backed by robust institutional structures for implementation (this is largely the EU’s strategy for increasing public acceptance of green biotechnology); threats of escalating trade disputes over differing regulations to force open foreign markets for the technology (a strategy favored by parts of the US government, the US biotech industry, and US farmers)
whereas nonoptimal private policies include
educating consumers about the benefits and (low) risks of the technology; highlighting consumer benefits of future GE products; ad hoc efforts to accommodate consumer demand for non-GE products through market driven product differentiation (crop segregation and labeling); lobbying the US government to force open foreign markets via trade disputes.[129]
Regulatory polarization is troubling for GM (or, if you like, GE) products because it “locks in or even increases fragmentation of international agricultural markets, and it implies reduced market access for agri-biotechnology and its products. It thus reduces scale economies and returns on investment into the technology.”[130] This state of affairs also “exerts a chilling effect” on adoption because of concerns about “market access for GE products.”
Though clean meat is far away from being affected by the sort of regulatory polarization that has settled into place around GM food, the prospect is not unthinkable, especially if one or more governments bring forward clean meat regulations earlier than expected.
Bernauer offers suggestions for digging out of the holes described above: “policy reforms that could help to avoid the seemingly unavoidable trajectory that leads from regulatory polarization to trade conflict to stagnation or decline of agri-biotechnology [should] focus on establishing strong regulatory authorities backed by robust liability laws, market-driven product differentiation based on mandatory labeling of GE products, and support for developing countries.”[131] The support for mandatory labeling stands out. It is certainly at odds with the positions of hard-charging 1990s biotechnology firms like Monsanto (but more in line with earlier companies like Calgene and Zeneca).
What does Bernauer mean by “policy reforms [that] focus on three elements: strengthening regulatory authorities and liability laws, supporting market driven product differentiation, and supporting developing countries”?[132] The European Union, Bernauer argues, would be best served by moving from decentralized, “network-like regulation” to establishing “powerful, politically independent, and science-oriented regulatory authorities.” The absence of such reform means increasingly complex regulation and further declines in public trust. Ideally, this would be accompanied by strengthened liability laws to improve public trust. “Public and private stakeholders,” meanwhile, should push for product differentiation, e.g. “national and international markets where GE and non-GE products can be safely and reliably traded.”[133] Finally, by “supporting developing countries,” Bernauer means that “international funding and technical support will be required to set up effective regulatory systems in developing countries, including also biosafety measures for R&D. Biotech accidents in developing countries could have disastrous implications for the technology in rich and poor countries. In addition, weak regulation in developing countries could hamper those countries’ agricultural export opportunities in markets subject to stricter and more effective agri-biotech rules.”[134]
Obviously, clean meat is a long way off from mid-stage considerations such as these.[135] However, it is certainly plausible that issues like regulatory polarization and trade conflicts will one day slow the adoption of clean meat, particularly as it moves from first-generation markets like the United States, Japan, and Western Europe to the rest of the world.
What if regulatory backlash does occur?
Bernauer suggests that for GMOs “to become profitable under a mandatory labeling or IP [identity preservation][136] system, several conditions [or a sufficient combination thereof] would have to be met.” Bernauer lists four:
- “[P]rices of GE seeds and related agrochemical products would have to decrease. Such a development is quite likely because of technological innovation and scale economies.” This development appears likely for clean meat as well: virtually all actors working on clean meat predict falling costs because of technological advances and experience curve effects. Whether costs could fall rapidly enough to overcome regulatory barriers is another matter, and a bootstrap paradox (or, we could say, a chicken-and-egg situation) may set in: for costs to come down, production must scale, and for production to scale, costs must decline to the point where the product is in heavy demand.
- “Yields of GE crops,” Bernauer adds, “would have to increase. This is quite possible.” This is analogous to increasing the output of clean meat production facilities per unit of capital and labor used—their productivity, in essence. Increases here remain likely, though contingent on technical details of clean meat production processes.
- “[L]abeling and IP [identity preservation] costs would have to decrease,” Bernauer writes. “Again, this is possible if large quantities are traded and processed and new technologies make IP and labeling more cost efficient.” This point would depend upon the particular regulatory requirements applied to clean meat. Labeling and IP are likely to be easier to implement in clean meat than in GM products, as bulk commodities (like corn and soybeans) from different sources are commonly mixed in the handling and shipping process in a way that clean meat products may not be. For example, it is difficult (although not impossible) to envision cultured meat being mixed with slaughtered meat at any point in the handling process.
- GM goods “would have to involve health or environmental quality traits that are so obvious to consumers that they become willing to pay a premium that cancels out IP and higher input costs. This would imply a reversal of current conditions, where premiums are paid for non-GE foods, and where existing GE foods are neither decisively cheaper nor of superior quality.”[137] Many clean meat products have a prospective advantage here because they are likely to possess “health or environmental quality traits” superior to slaughtered meat. Whether these traits will become “obvious to consumers” remains another matter. It could be advantageous for clean meat to enter markets from the top, as a premium alternative to slaughtered meat. Competing in the premium category has the advantage of mitigating perceptions of clean meat as a cost-cutting or efficiency measure (the way GMOs were sometimes seen) and of raising price parity targets for beef from about $10.91/kg (Consumer Reports’ average price paid for conventional beef) to about $17.26/kg (Consumer Reports’ average price paid for organic grass-fed beef).[138] (Other meats show similar price disparities between conventional and premium products.)
While clean meat currently faces no regulation comparable to that faced by GMOs, the US Cattlemen’s Association letter to the USDA and a provision in a US House spending bill giving the USDA authority to regulate clean meat[139] represent the first skirmishes over some form of labeling and identity preservation.
Summary of implications and findings
- Attitudes of secrecy and arrogance (or the widespread perception thereof) by large GMO producers, especially Monsanto from the mid-1990s to today, hurt the adoption of GM food. Monsanto’s “storming” of European markets in the late 1990s proved especially damaging.
- However, the popular view that GMOs’ controversiality represent an overreach characteristic of large, corrupt “megacorporations”[140] like Monsanto and that newer food technologies like clean meat will avoid these controversies because they are being developed by smaller, socially-conscious startups is misguided. The history of gene editing commercialization can be broadly divided into two periods. The startup phase begins with the founding of Genentech in 1976 and extends into the mid-1990s. Its heyday comes with Calgene and Zeneca successfully bringing GM tomatoes and tomato paste to market in 1994 and 1996. It is marked by optimism, large amounts of external funding, rapid growth, and few commercial products. The startup phase melts into the second, corporate phase via rounds of mergers and acquisitions in the 1980s and 1990s. (Calgene and Zeneca are sold off and merged in 1997 and 1999, for example.) It is marked by larger, established firms like Dow Chemical and Monsanto attempting to bring products to market and to set up profit structures around their intellectual property. Today’s clean meat (and plant-based meat) firms are not latter-day Monsantos with better ethics: they are latter-day Calgenes and Zenecas, similar in company culture, ethics, funding needs, and facing the challenge of getting a profitable product to market without running out of funding. The industry structure of early biotechnology firms resembles the industry structure of early clean meat research (small startups who are beginning to attract the notice of large, established firms). Moreover, the attitudes, vision, and stated aims of the researchers involved in biotechnology from the 1970s through the 1990s resemble those of clean meat researchers and advocates today.
- One additional implication of the two-phase industry model is that clean meat adoption could be radically changed if the industry underwent a round of mergers and acquisitions similar to biotechnology in the 1990s. It is possible that larger firms could scale clean meat products more effectively. It is also possible that the (real or perceived) corporatization of clean meat production would provoke significant backlash and slow or reverse adoption. In the case of GM food in Europe, the second factor probably outweighed the first. In the United States, the first factor probably outweighed the second. This suggests that industry consolidation poses less of a risk in the United States than it does in Europe.
- Much of the successful activist action against GM food came in the form of relatively small campaigns focused directly on companies (especially those occupying vulnerable positions in a supply chain). Comparatively less direct change came about via changing public opinion first then using that broad base of support to effect change. Focused campaigns, even if relatively small, were more influential than broad changes in public opinion.
- Unwillingness to regulate GMOs in a timely manner may have soured the public more than they otherwise would have been on GM food. Monsanto's head of regulatory affairs, Leonard Guarraia, argued that anti-regulation FDA spokesman Henry Miller “did more harm to biotechnology than [anti-GMO activist] Jeremy Rifkin ever did. He put the government completely at odds with the critics.” Will Carpenter adds that Miller “thought he was helping us. But I told him that we couldn’t stand much more of his help.”[141] Rifkin himself, quoted in the New York Times, said that “If the F.D.A. had required tests and labels, ‘it would have been more difficult for us to mobilize the opposition.’”[142] This suggests that sensible regulation can minimize delays in adoption and maximize the alleviation of public and opposition concerns and is therefore preferable to no regulation or lax regulation.
- Many early experts on GM food technology predicted a future in which applied genetic engineering had solved major problems in agriculture, nutrition, sustainability, and food security. Many of their evaluations match, sometimes word-for-word, current prognostication around clean meat. However, virtually none of the world-changing GMO predictions came to pass.[143] Clean meat experts, even (or especially) those familiar with the technology, should be wary of any consensus view that claims that clean meat will transform the global food system in this or that radical way. It is unclear whether the optimistic statements from GM food experts hurt or helped their cause, but insofar as they believed those statements, the incorrect predictions might have led to other strategic mistakes.
- In the same way that no GM utopias arose, none of the apocalyptic predictions about GM food came true. Many biotech researchers would remark that this was no surprise, because worries of apocalypse were ludicrous to begin with. Ludicrous or not, biotech companies should have taken opposition to GM food more seriously. Many of the most obvious blunders (e.g., Monsanto’s strategic decisions in the second half of the 1990s) could have been avoided by taking activist concerns, public fears, and the cultural differences between markets seriously. What works in Nebraska may provoke disgust in Brittany and indifference in Sichuan. The story of GM food indicates that effective strategies for encouraging adoption were not that hard to develop, but convincing key firms to act in a strategic way to begin with was in fact quite difficult.
- Clean meat already faces concerns about unnaturalness. The history of new technologies, however, indicates that concerns around unnaturalness alone are not sufficient to provoke widespread backlash (or else many prescription drugs and medical interventions would go unused). The risk of backlash is highest, rather, when concerns from different areas overlap and intensify one another (e.g., corporate control of food meets unnaturalness). Concerns about unnaturalness could be significantly greater for food than for other applications like medicine. However, this rule is not absolute: society has adopted pasteurization, antibiotics and hormones fed to farmed animals, and numerous other widespread food technologies while some medical technologies seen as unnatural, like cloning and vaccines, have provoked opposition.
- Clean meat companies should exercise caution around aggressive patenting and intellectual property protection. Any move that could be interpreted as enforcing “patents on life” could be especially damaging to public opinion. Decisions by biotechnology companies, particularly Monsanto, to defend patents on, for example, Roundup Ready soybean seeds by suing farmers for replanting these seeds has contributed to the view that GMOs are at bottom a tool for agricultural firms to control the world’s food supply.[144]
- The framing of an issue often overwhelms underlying technical or economic facts, so paying attention to the way a new technology is being interpreted and understood remains important even if the benefits of that technology seem obvious and the drawbacks inconsequential. Public discussion often has the effect of rendering benefits inconsequential and dangers bottomless. (Or: benefits abstract and far, dangers personal and close.) Being strategic about framing remains hugely important. For an example, see Zeneca and Calgene’s marketing of their products as GM.
- Focusing on the positive aspects of a technology has been more successful than publicly responding to negative perceptions. Zeneca and Calgene’s marketing of their tomato products as GM and stronger for it succeeded in a way later public relations strategies around GM food did not. The examples of Zeneca and Calgene reinforce the value of focusing on the positive aspects of a new product rather than endlessly rebutting fears and negative perceptions. The limits of a rebutting strategy were on display in debates over the adoption of nuclear power in France, the US, and elsewhere. Constant discussion of safety concerns, even if to answer them in a technically-sound manner, tends to replace positive frames of an issue with frames that center on whether a technology will cause colon cancer or annihilate one’s children—even if there is little evidence that these concerns are warranted. This dynamic is exacerbated by the fact that non-experts often make decisions based on acceptability rather than risk, so a technical totting-up of the relative risks and benefits of a technology is likely to be subsumed to a reactive acceptability/non acceptability binary in public discourse.
- The foregoing point about positive framing invites questions around whether clean meat companies should tout their product’s superior food safety over slaughtered meat. Discussing clean meat’s safety (even if to point out that it is good) could open up issues of safety and risk in a way that is inadvisable. However, it seems hard to recommend avoiding the topic of safety altogether when it may become a point of considerable contention and when clean meat may offer significant advantages in this area. It is likely more effective to frame clean meat in terms of the existing risks of slaughtered meat. Messaging should bear less resemblance to headlines like “Why clean meat is safe” and more resemblance to headlines like “Why clean meat is safer than slaughtered meat.” This messaging shift is also advisable when communicating with oppositional groups focused on health or environmental risks, because these groups are often suspicious of positive claims and receptive to negative claims or criticisms of existing institutions.
- A public setback in one market can lead to cascading rejection in other markets, as happened in parts of Africa and Asia after Europe’s 1998 moratorium on GM crops.
- Food safety incidents may have an ambiguous impact on clean meat reception. On one hand, tainted meat scandals tend to hurt sales and public perceptions of slaughtered meat.[145] If clean meat can position itself as a safer alternative to slaughtered meat, it could be adopted more rapidly as a result of tainted-meat fears. This scenario is not without risk, however: food safety scares render the public more fearful and likely less willing to trust new food in general.
- Whether because of causation or other reasons for correlation, consumers “in countries with mandatory positive labeling [for GM food] are usually also more critical of the technology.”[146] Various attempts to impose de facto labeling requirements on clean meat have already been made by cattlemen’s associations and lawmakers. It is possible that clean meat manufacturers could proactively differentiate their products from slaughtered meat, sidestepping labeling concerns. Because appearing to resist regulation risks public backlash, it might be effective for clean meat firms to resist proposed prohibitions on using the word “meat” but to offer, as an alternative, proactive labelling of their products as involving no animal slaughter, as from an animal but not part of an animal, or some other distinction. Labelling requirements may well happen in the absence of a proactive move, and clean meat companies have an interest in differentiating their products to, among other considerations, avoid the perception of sneaking into markets the way GMOs tried to.
- GMO producers may have been too slow to organize an industry group for themselves in the EU. Clean meat advocates should probably focusing on developing a robust industry advocacy group in each market they plan to enter.
- Supply chain dynamics are likely to influence clean meat adoption. The wave of European supermarkets dropping GM ingredients in the late 1990s was intensified by a highly competitive retail environment, by a supply chain structure in which sellers were susceptible to pressure from buyers, and by the failure of American biotechnology firms to secure buy-in from European processors, handlers, and retailers. Securing buy-in from retailers and other distributors has already been a matter of consequence for plant-based meat companies like Impossible Foods and Beyond Meat. It is too early to tell if Impossible and Beyond products will resemble Calgene’s Flavr Savr, a novel product from a young company that sold well and generated interest before being discontinued, or will transform into scalable elements of the food supply.
- Opposition to GM food arose as one component of a broader set of societal concerns relevant in the rich democracies from the 1960s on. If opposition to clean meat develops, it is almost certain to grow within a matrix of intersecting causes and identities, some of which may seem oddly paired. An opposition coalition could draw support from sources as diverse as deep green environmentalism, cattlemen’s associations, organic farming operations, urban foodies interested in “authenticity,” factory farm operations, farm workers’ unions, deontologically-inclined vegans, deontologically-inclined carnivores, and so forth.
- If clean meat does face regulatory impediments and public backlash, a variety of remedies currently prescribed for GM food could work to break up an impasse. These include efforts to build public trust by strengthening (rather than weakening) food regulators and liability laws, reducing production costs and improving production efficiency, increasing perceived consumer benefit, and a renewed focus on developing markets.
Appendix one: United States public opinion polling on biotechnology and genetically modified food
The following table is organized broadly by chronology, but with an eye toward keeping identical questions together. Although this table focuses on the period from 1994 to 2003, when the GMO debate was in a crucial formative period (and before which little polling exists), one contemporary poll (from 2016) is included for context.
Question or issue | GMO friendly or open responses | GMO hostile or wary responses | Year | Notes |
“Company A manufactures… some food products that have genetically engineered ingredients and some products which have all natural ingredients. Company B manufactures… only food products which have all natural ingredients. If the products were… less expensive from Company A, would you buy products from Company A, from Company B, from both or would it not make any difference to you?”[147] | 26% (Company A) | 27% (Company B) | 1994 | 17% said both and 27% said it made no difference. |
“If you were to learn that a product you frequently use was being genetically engineered, would you… not worry about it and keep using the current product, look for another brand in the product category that is not genetically engineered, or stop using that type of product altogether?” | 38% (keep using) | 55% (look for another brand + stop using responses aggregated) | 1994 | Tendentious question phrasing suggests to consumers that there’s something to worry about. |
“Perception of genetic engineering as a serious health hazard”[148] | - | 21% viewed genetic engineering as a serious health hazard | 1995 | Versus 65% in Sweden (highest), 57% in Germany, 39% in the UK, 28% in Norway (lowest). |
“Willingness to buy biotechnology produce developed through biotechnology to resist insect damage” | 73% said they were willing | - | 1995 | Versus 74% in Canada (highest), 63% in the UK, 30% in Germany, 22% in Austria (lowest). |
“GM food will bring benefits to a lot of people”[149] | 65.4% (strong + moderate agreement) | 21.2% (strong + moderate disagreement) | 2000 | |
“Do you think genetically modified foods are basically safe, basically unsafe, or don't you have an opinion on this?” | 29% (strong + moderate safe) | 24% (strong + moderate unsafe) | 2001 | |
“Now, as you may know, more than half of the products at the grocery store are produced using some form of biotechnology or genetic modification. Knowing this, do you think genetically modified foods are basically safe, basically unsafe, or don't you have an opinion on this?” | 47% (strong + moderate safe) | 22% (strong + moderate unsafe) | 2001 | Note minor phrasing difference with above and its large effect on responses. |
“GM food is fundamentally against nature” | 44.2% (strong + moderate disagreement) | 46.9% (strong + moderate agreement) | 2000 | |
“Scientists can change the genes in some food crops and farm animals to make them grow faster or bigger and be more resistant to bugs, weeds and disease. Do you think this genetically modified food, also known as bio-engineered food, is or is not safe to eat?”[150] | 35% (safe) | 52% (unsafe) | 2001 | |
[same as above] | 46% (safe) | 46% (unsafe) | 2003 | |
“Do you favor or oppose scientific research into genetic modifications of food?”[151] | 65% (strong + weak support) | 25% (strong + weak opposition) | 2001 | |
“Do you think it's right or wrong to use scientific techniques to do things like enhance the flavor and nutrients, or prolong the freshness of food?”[152] | 65% (right) | 32% (wrong) | 1999 | |
“Overall would you say you strongly support, moderately support, moderately oppose, or strongly oppose the use of biotechnology in agriculture and food production?”[153] | 48% | 41% | 1999 | |
[same as above] | 51% | 41% | 2000 | |
[same as above] | 52% | 38% | 2001 | |
[same as above] | 48% | 45% | 2002 | |
[same as above] | 45% | 45% | 2005 | |
“Overall do you think the benefits of developing and growing these new (genetically modified) plants and crops outweigh the risks of doing this, or do you think the risks outweigh the benefits?”[154] | 38% (benefits outweigh risks) | 48% (risks outweigh benefits) | 2000 | |
“I believe that in the long run, the potential benefits of genetically modified foods will outweigh the potential risks.”[155] | 51% agree | (Not reported.) | 1998 | |
[same as above] | 43% agree | 48% disagree | 2000 | |
“Foods with genetically modified ingredients are generally ____ than foods with no genetically modified ingredients.”[156] | 39% (neither better nor worse for health + better for health responses) | 33% (worse for health) | 2016 | Of 39% positive, 32% selected neither better nor worse and 7% selected better. |
Additional data from surveys conducted in Europe and the United States in 1996 and 1997:
Data and table from Gaskell, “Worlds,” 385.
Appendix two: Selected accounts of early attitudes within biotechnology
[T]he great biotechnology craze of 1979 and 1980… washed across the American business landscape like a giant wave. It began in the summer of 1979 with the announcement that scientists had managed to splice a useful gene—the human gene that produced the body's growth hormone—into bacteria, turning those bacteria into factories for the precious hormone.
Murmurs of an impending revolution grew in volume on June 16, 1980, when the U.S. Supreme Court ruled that living organisms created by the human hand—in this case, a genetically altered microbe—could be patented. The case had been in the works since 1972; a researcher named Ananda Chakrabarty, working at General Electric's Schenectady laboratories, had managed to squeeze genes from one type of bacterium into another, creating a new strain that promised to be useful in cleaning up oil spills. The methods Chakrabarty used quickly became obsolete, but by the time the case bearing his name arrived at the Supreme Court, fortunes were riding on the outcome.
Patent applications covering techniques for modifying bacteria, and the modified bacteria themselves, had been piling up in the chemical division of the patent office. Patent officials refused even to examine them until the Chakrabarty case was decided.
The Supreme Court decision “was a signal that this industry was going to be recognized. And intellectual property rights were going to be recognized,” says Kate Murashige, a lawyer and pioneer in gene patenting, who worked for Genentech, one of the original biotech startup companies, in the early 1980s. “The management of Genentech, when I worked there, was convinced that, were it not for patents, they could not survive as a company. It was always considered an essential part of the business plan.”
Soon after, the first biotech bull market roared its assent. On October 14, 1980, Genentech offered a million shares of stock for sale at $35 a share. Frenzied buyers bid the price up to $89 within hours.By the end of the day, the company was worth half a billion dollars. It still didn’t have a product to sell.
The scientific heart of this first biotech boomlet lay in California,along the San Francisco Bay, in the laboratories at Stanford and the University of California. The boomlet’s spiritual heart lay there too, in a place where a great tide of explorers washed up against the continent’s western coast, stared at the day’s dying light, and contemplated new frontiers beyond the merely geographic. Certainly, small companies devoted to agricultural biotechnology also emerged elsewhere, in Colorado, Wisconsin, and Texas. Yet the early prophets of biotechnology did fit the California stereotype. They were restless and enthusiastic. Sometimes they blithely disregarded cautionary lessons of experience and history. And they had one other significant thing in common: They were relative strangers to agriculture. They promised to transform a world that they barely understood.
“All things seemed possible,” says Peter Carlson, who cofounded a small company called Crop Genetics International in 1981. “For the first time, a good story was as important as performance in the marketplace.” He adds, with a grin: “It’s easier to weave dreams when you don’t know the roadblocks ahead.”
It was indeed the day of the dream weavers. Among them was David Padwa, a precocious child of New York City who'd made his first few millions in the computer business before he dropped out during the 1960s, traveled the world, and dabbled in environmental causes. He spent 1981 on the road, talking to potential investors, pitching the dream of an agricultural revolution. He raised $55 million and set up a company called Agrigenetics. Admiring reporters wrote that Agrigenetics would have “miracle crops” in hand within five years.
In San Carlos, California, scientist Martin Apple received a stream of visitors at another fledgling company, the International Plant Research Institute, or IPRI. “We are going to make pork chops grow on trees,” Apple told the New York Times. When that quote appeared in the newspaper, Apple was mortified. He meant, of course, that engineered plants might produce the same nutrients that one finds in a pork chop, not an actual hunk of meat hanging on a tree. Besides which, as an observant Jew, he'd never touched a pork chop in his life. He called the chairman of his board, asking how they might get the Times to print a correction. The chairman was amused. “Don't worry about it,” he told Apple. “It's great publicity.”
Charles, Harvest, 10-12.
These young genetic engineers did believe that their work would be good for the planet, possibly making it easier to grow food or reducing agriculture's dependence on chemicals. Some of them, working inside chemical companies, often saw themselves as “green” revolutionaries fighting against the entrenched power of the chemists… They’d seen DDT banned and Earth Day celebrated. Chemicals represented a dirty and regrettable past, and biology was the savior.
At Monsanto those views “came from the very top,” says Pam Marrone, a researcher at Monsanto during the late 1980s. “I remember having lunch with [then-CEO] Dick Mahoney and him saying, ‘Because of parathion [a particularly hazardous insecticide], I don’t ever want to be in chemicals again. And that’s why we’re in biotechnology.’”
“During those years, all of us who went into biology were influenced by the wave of environmentalism,” says Willy de Greef, who worked… for Plant Genetic Systems [and] Novartis.
Charles, Harvest, 24-25.
For a few years in the early 1980s, the pioneers of genetic engineering were simply caught up in the fun of it all. They sometimes spoke as though genes were becoming mere playthings in their hands.
Mary-Dell Chilton abandoned the academic world. In the spring of 1983, she left St. Louis for Research Triangle Park, North Carolina, where she set up a new biotechnology operation for Ciba-Geigy, the Swiss chemical giant. “The solutions are coming very fast now,” she told Business Week in 1984. “In three years, we'll be able to do anything that our imaginations will get us to.”
Ernie Jaworski dubbed the fourth floor of U Building, the lair of Monsanto's genetic engineers, U-4ia. The spirit of the place was indeed euphoric. Many of those who worked there look back on the years 1983 to late 1985 as a kind of golden age. They felt—they knew—that, when it came to knowledge about the inner workings of a plant’s genetic machinery, they lived at the center of the scientific universe.
Charles, Harvest, 31.
Harry Klee… arrived at Monsanto in 1984. “I had sworn I would never work in industry,” Klee recalls. “But when I got to Monsanto, it was just instantly apparent that if I wanted to do plant biotechnology, this was the place to be.” It wasn't just that Monsanto offered superior resources, Klee says. Paradoxically, it was also a much more collegial place. In academia every colleague is also a competitor; every collaboration involves a negotiation over credit. At Monsanto, Klee says, much of that was stripped away. “There was less ego involved."
The genetic transformation of plants rapidly became routine. Genetically altered petunia plants filled the laboratory with a splendid array of colors. Those petunias remain Jaworski's strongest memory of that time; it was a “thrill,” he says, “knowing that all of them had our genes in them.”
“Anything was worth doing because it was new,” says Steve Rogers. Almost every conceivable question needed answering.
Charles, Harvest, 32.
When molecular biologists, geneticists, and plant biochemists first developed the ability to cut and splice genes from one organism into another in the 1970s and 1980s, the prospects for this revolutionary new technology looked remarkably open and bright. The scientific profession, the media, venture capital, and Wall Street were abuzz with the possibilities these new “recombinant DNA” technologies held out for generating a whole new industrial frontier and for solvinghost of agriculture and health-related problems. For these enthusiasts,the new biotechnologies offered a novel way to shortcut the slow process of traditional plant and animal breeding, to raise agricultural productivity, and to make better and cheaper medicines, all while representing potentially enormous source of profit for the firms involved. In the tremendous excitement of this first stage, the biotech scientist-entrepreneurs saw their work as an enterprise in which “everybody wins.”
Their enthusiasm was infectious. Large corporations and finance capitalists poured money into these new ventures and built a massive scientific-cum-business infrastructure dedicated to generating new discoveries and new products with recombinant DNA. Indeed, when genetically engineered crops were introduced into the market in 1996, they took off at a phenomenal rate. The first crops planted commercially were corn,soybeans, canola, and cotton. By the time of the FDLI conference [in 2001], global plantings of these crops had grown from 4.2 million acres in six countries to 130 million acres in thirteen countries, a thirtyfold increase. Some observers hailed this as the most rapid uptake of a new technology in human history. For many, the “gene revolution” of transgenic technology would underpin a second Green Revolution to resolve the challenge of global hunger.
Schurman and Munro, Fighting, xi-xii.
[M]any of the high-level executives of these multinational corporations believed that whatever opposition arose to these new technologies could be effectively managed through a (corporate-designed) policy of government regulation and public education. From their perspective, the naysayers who failed to acknowledge the benefits of biotechnology were simply anti-technology “neo-Luddites” or environmental extremists who were unlikely to garner much sympathy among policymakers or the general public. For their part, the scientists in these companies (and some of the managers) were so convinced of the transformative power and social and environmental benefits of genetic engineering that they could not imagine that social opposition to these technologies would seriously grow or become significant in some other way. Consequently, both groups tended to discount the legitimacy and the import of the opposition.
Schurman and Munro, Fighting, 17.
Agricultural scientists enthusiastically embraced the new biotechnologies because they offered enormous potential to improve agricultural productivity. Often trained in U.S. land grant universities, these scientists took for granted the idea that improving agricultural productivity and addressing (mainly U.S.) farmers' problems were the ultimate goals of agricultural research. Genetic engineering was merely a way of augmenting yields and addressing the problems of agriculture more quickly and efficiently than had been possible using traditional methods of plant and animal breeding. Furthermore, in many scientists’ minds, the new methods of gene transfer were more precise. “We were only putting one gene in, and we know exactly where it went,” noted a specialist in plant virology. “In the past we played roulette. We now have control over where the ball lands,” noted another biotechnology researcher.
As most bioscientists saw it, genetic engineering was simply one more in a long line of advances in the way human beings produce their food. “My basic premise is that genetic technology is simply a continuation of all other aggregate technology in agriculture,” explained one scientist turned-biotech entrepreneur.
It started [long] ago [with] tractors, fertilizer, herbicides, insecticides,labor-saving devices, refrigeration, transportation, and new varieties, and in recent decades, genetic alterations using transgenic approaches.... I don't see that, from the corporate perspective, there was any difference at the value of biotechnology at the time. This was just another way to improve productivity.
Schurman and Munro, Fighting, 27.
The course of this technology has been altered significantly, and its future, once so clearly envisioned by its proponents, is far less assured.
Schurman and Munro, Fighting, 183.
Selected bibliography
“A Bill Making appropriations for Agriculture, Rural Development, Food and Drug Administration, and Related Agencies programs for the fiscal year ending September 30, 2019, and for other purposes.” 2018. https://docs.house.gov/meetings/AP/AP01/20180509/108287/BILLS-115HR-SC-AP-FY2019-Agriculture-SubcommitteeDraft.pdf.
Aggarwal, Saurabh. “What's fueling the biotech engine—2011 to 2012.” Nat. Biotechnol. 30 (2012): 1191–1197.
B Dasarath Reddy. “Grey market corners new GM hybrids as farmers look beyond Bt cotton.” Business Standard, July 24, 2017. http://www.business-standard.com/article/economy-policy/grey-market-corners-new-gm-hybrids-as-farmers-look-beyond-bt-cotton-117072400399_1.html.
Berg, Paul et al. “Summary Statement of the Asilomar Conference on Recombinant DNA Molecules,” Proc. Natl. Acad. Sci. 72, no. 6 (1975): pp. 1981-1984.
Bernauer, Thomas. Genes, Trade, and Regulation: The Seeds of Conflict in Food Biotechnology. Princeton: Princeton University Press, 2016.
Biotech Crop Highlights in 2016. International Service for the Acquisition of of Agri-biotech Applications. Accessed March 16, 2018. http://www.isaaa.org/resources/publications/pocketk/16/.
Bonny, Sylvie. “Why are most European opposed to GMOs? Factors explaining rejection in France and Europe.” Electronic Journal of Biotechnology 6, no. 1 (2003).
Charles, Daniel. Lords of the Harvest: Biotech, big money, and the future of food. Cambridge, MA: Perseus, 2001.
Eichenwald, Kurt. “Redesigning Nature: Hard Lessons Learned; Biotechnology Food: From the Lab to a Debacle.” New York Times, January 25, 2001.
“EU court annuls approval of BASF's Amflora GMO potato.” Reuters. December 13, 2013. https://www.reuters.com/article/eu-gmo-potato/eu-court-annuls-approval-of-basfs-amflora-gmo-potato-idUSL6N0JS1TH20131213.
Fedoroff, Nina and Nancy Marie Brown. Mendel in the Kitchen. Washington, D.C.: Joseph Henry Press, 2004.
Fife-Schaw, Chris, and Gene Rowe. “Extending the application of the psychometric approach for assessing public perceptions of food risk: some methodological considerations.” Journal of Risk Research 3, no. 2 (2000): 167–179.
Fraley, Robert et al. “Expression of bacterial genes in plant cells,” Proc. NatL. Acad. Sci. USA 80 (1983): 4803-4807.
Friedrich, Bruce. “‘Clean Meat’: The ‘Clean Energy’ of Food.” Good Food Institute, September 6, 2016. http://www.gfi.org/clean-meat-the-clean-energy-of-food.
Gaskell, George, et al. “Biotechnology and the European public.” Nature Biotechnology 18 (2000): 935-38.
Gaskell, George, et al. “Worlds Apart? The Reception of Genetically Modified Foods in Europe and the U.S.” Science 285 (1999): 384-87.
“Global Status of Commercialized Biotech/GM Crops: 201.,” ISAAA Brief 52. May 2017. https://www.isaaa.org/resources/publications/briefs/52/download/isaaa-brief-52-2016.pdf.
Goeddel, D. V. et al. “Expression in Escherichia coli of chemically synthesized genes for human insulin,” Proc. Natl. Acad. Sci. USA 76, no. 1 (1979): 106–110.
The Good Food Institute. “RE: U.S. Cattlemen’s Association Petition to Restrict Beef and Meat Terms on Food Labels.” Response to FSIS-2018-0016. April 17, 2018. http://www.gfi.org/images/uploads/2018/04/GFIetal-Comment-FSIS-2018-0016.pdf
Goodell, Rae. “Public Involvement in the DNA Controversy: The Case of Cambridge, Massachusetts.” Science, Technology, & Human Values 4, no. 27 (1979): 36-43.
Herring, Ronald. “Epistemic brokerage in the bio-property narrative: contributions to explaining opposition to transgenic technologies in agriculture.” New Biotechnology 27, no. 5 (2010): 614-622.
Herring, Ronald. “Opposition to transgenic technologies: ideology, interests and collective action frames.” Science and Society 9 (2008): 458-63.
Herring, Ronald and Robert Paarlberg. “The Political Economy of Biotechnology.” Annual Review of Resource Economics 8 review in advance (2016): 8.1-8.20.
Hoban, Thomas J. “Consumer acceptance of biotechnology: An international perspective.” Nature Biotechnology 15 (1997): 232-34.
Hofstede, G. Culture’s Consequences: Comparing Values, Behaviors, Institutions, and Organizations Across Nations. London: Sage, 2001.
Jackson, D. A., R. H. Symons, and Paul Berg. “Biochemical Method for Inserting New Genetic Information into DNA of Simian Virus 40: Circular SV40 DNA Molecules Containing Lambda Phage Genes and the Galactose Operon of Escherichia coli.” PNAS 69, no. 10 (1972): 2904–2909.
James, Clive. “Global Status of Transgenic Crops in 1997,” ISAAA Briefs no. 5 (1997).
Jasanoff, Sheila. Designs of Nature: Science and Democracy in Europe and the United States. Princeton, NJ: Princeton University Press, 2005.
Kuntz, Marcel. “The GMO case in France: Politics, lawlessness and postmodernism.” GM Crops & Food 5, no. 3 (2014): 163-169.
Missouri State House. House Bill 2607, 2018. https://house.mo.gov/billtracking/bills181/sumpdf/HB2607C.pdf.
Mohorčich, J. “What can nuclear power teach us about the institutional adoption of clean meat?” Sentience Institute. November 28, 2017. https://www.sentienceinstitute.org/nuclear-power-clean-meat.
More Americans Willing to Ride in Fully Self-Driving Cars. AAA NewsRoom. January 24, 2018. http://newsroom.aaa.com/2018/01/americans-willing-ride-fully-self-driving-cars/.
Morrow, J. F., S. N. Cohen, A. C. Chang, Herbert Boyer, H. M. Goodman, R. B. Helling. “Replication and transcription of eukaryotic DNA in Escherichia coli.” Proceedings of the National Academy of Sciences of the United States of America 71, no. 5 (1974): 1743–47.
National Farmers Union. NFU Statement on Proposed Senate GMO Labeling Bill. February 23, 2016. https://nfu.org/2016/02/23/nfu-statement-on-proposed-senate-gmo-labeling-bill/.
Paarlberg, Robert. “The Global Food Fight.” Foreign Affairs 79, no. 3 (2000): 24-38.
Paarlberg, Robert. Starved for Science. Cambridge: Harvard University Press, 2008.
Paarlberg, Robert. “Strengthening NSF Sponsored Agribiotechnology Research.” Statement to the U.S. House of Representatives Committee on Science, September 25, 2001. http://www.agbioworld.org/newsletter_wm/index.php?caseid=archive&newsid=1196.
Same-Sex Marriage and Gay Rights: A Shift in Americans’ Attitudes. The Associated Press-NORC Center for Public Affairs Research. May 5 2015. http://www.apnorc.org/projects/Pages/HTML%20Reports/same-sex-marriage-and-gay-rights-a-shift-in-americans-attitudes0305-8272.aspx.
Schurman, Rachel. “Fighting ‘Frankenfoods’: industry opportunity structures and the efficacy of the anti-biotech movement in Western Europe.” Social Problems 51, no. 2 (2004): 243–68.
Schurman, Rachel, and William Munro. Fighting for the Future of Food. Minneapolis: University of Minnesota Press, 2010.
Sorenson, A. W., et al. “Impact of 'Mad Cow Disease' publicity on trends in meat and total vitamin A consumption in Geneva between 1993 and 2000.” European Journal of Clinical Nutrition 57, no. 1 (2003): 177-85.
US Cattlemen’s Association. “PETITION FOR THE IMPOSITION OF BEEF AND MEAT LABELING REQUIREMENTS: TO EXCLUDE PRODUCTS NOT DERIVED DIRECTLY FROM ANIMALS RAISED AND SLAUGHTERED FROM THE DEFINITION OF ‘BEEF’ AND ‘MEAT.’” February 9, 2018. https://www.fsis.usda.gov/wps/wcm/connect/e4749f95-e79a-4ba5-883b-394c8bdc97a3/18-01-Petition-US-Cattlement-Association020918.pdf?MOD=AJPERES.
Winerip, Michael. “You Call That a Tomato?” New York Times. June 24, 2013. http://www.nytimes.com/2013/06/24/booming/you-call-that-a-tomato.html.
[1] Daniel Charles, Lords of the Harvest: Biotech, big money, and the future of food (Cambridge, MA: Perseus, 2001), 212.
[2] I.e. GM foods, transgenic foods, GMO foods, etc. While terms like “genetically modified” and “transgenic” are not scientifically identical, these differences end up playing virtually no role in the public perception and adoption of genetically modified food.
[3] The limits of a rebutting strategy came to the fore in debates over the adoption of nuclear power in France, the United States, and elsewhere.
[4] “We… developed methods for covalently joining duplex DNA molecules to one another and… used these techniques to construct circular dimers of SV40 DNA and to insert a DNA segment containing lambda phage genes and the galactose operon of E. coli into SV40 DNA. The method involves: (a) converting circular SV40 DNA to a linear form, (b) adding single-stranded homodeoxypolymeric extensions of defined composition and length to the 3′ ends of one of the DNA strands with the enzyme terminal deoxynucleotidyl transferase (c) adding complementary homodeoxypolymeric extensions to the other DNA strand, (d) annealing the two DNA molecules to form a circular duplex structure, and (e) filling the gaps and sealing nicks in this structure with E. coli DNA polymerase and DNA ligase to form a covalently closed-circular DNA molecule.” D. A. Jackson, R. H. Symons, and Paul Berg, “Biochemical Method for Inserting New Genetic Information into DNA of Simian Virus 40: Circular SV40 DNA Molecules Containing Lambda Phage Genes and the Galactose Operon of Escherichia coli,” PNAS 69, no. 10 (1972): 2904–09.
[5] J. F. Morrow, S. N. Cohen, A. C. Chang, Herbert Boyer, H. M. Goodman, R. B. Helling, “Replication and transcription of eukaryotic DNA in Escherichia coli,” Proceedings of the National Academy of Sciences of the United States of America 71, no. 5 (1974): 1743–47.
[6] For this reason, Boyer and Cohen ended up with the credit for creating the first genetically modified organism. Rudolf Jaenisch created the first GM animal by inserting DNA from another organism into a mouse embryo in 1974.
[7] “Although our assessments of the risks involved with each of the various lines of research on recombinant DNA molecules may differ, few, if any, believe that this methodology is free from any risk. Reasonable principles for dealing with these potential risks are: (i) that containment be made an essential consideration in the experimental design and, (ii) that the effectiveness of the containment should match, as closely as possible, the estimated risk. Consequently, whatever scale of risks is agreed upon, there should be a commensurate scale of containment. Estimating the risks will be difficult and intuitive at first but this will improve as we acquire additional knowledge; at each stage we shall have to match the potential risk with an appropriate level of containment.” Paul Berg et al., “Summary Statement of the Asilomar Conference on Recombinant DNA Molecules,” Proc. Natl. Acad. Sci. 72, no. 6 (1975): 1981-1984 (pages).
[8] D. V. Goeddel et al. “Expression in Escherichia coli of chemically synthesized genes for human insulin,” Proc. Natl. Acad. Sci. USA 76, no. 1 (1979): 106–110.
[9] Saurabh Aggarwal, “What's fueling the biotech engine—2011 to 2012,” Nat. Biotechnol. 30 (2012): 1191–1197.
[10] “[I]t should now be possible,” the researchers note in a prescient final paragraph, “by using Ti plasmids that have the tumor genes (i.e., tms and tmr loci, 12) deleted, to obtain kanamycin-resistant transformants that can be readily and reproducibly regenerated into phenotypically normal plants... there is no reason to believe that NPTase I and NPTase II are unique in their ability to be expressed in plant cells and it is quite likely that other bacterial, fungal, or mammalian genes, including those whose products could be expected to modify plant properties in a useful manner, could also be successfully engineered and expressed.” Robert T. Fraley et al., “Expression of bacterial genes in plant cells,” Proc. NatL. Acad. Sci. USA 80 (1983): 4803-4807.
[11] Clive James, “Global Status of Transgenic Crops in 1997,” ISAAA Briefs no. 5 (1997): 31.
[12] See Michael Winerip, “You Call That a Tomato?” New York Times, June 24, 2013, http://www.nytimes.com/2013/06/24/booming/you-call-that-a-tomato.html.
[13] “Global Status of Commercialized Biotech/GM Crops: 2016,” ISAAA Brief 52, May 2017, https://www.isaaa.org/resources/publications/briefs/52/download/isaaa-brief-52-2016.pdf.
[14] Ron Herring and Robert Paarlberg, “The Political Economy of Biotechnology.” Annual Review of Resource Economics 8 review in advance (2016): 8.3.
[15] Percentage of respondents saying they would buy GM produce designed to resist insect damage. See Thomas J. Hoban, “Consumer acceptance of biotechnology: An international perspective,” Nature Biotechnology 15 (1997): 232-34.
[16] “[Jeremy] Rifkin… focused on the consumer end of the commodity chain, circulating a letter to a dozen of the nation’s top supermarket chains, asking them to clarify their policies toward [genetically-engineered] rBGH milk for the public record…. Rifkin [also] informed these supermarket chains of a yet-to-be-published paper by a University of Chicago physician and professor of medicine that exposed the human health risks of the synthetic hormone [rBGH]. Anxious about the consumer reaction, spokespeople for Safeway, Kroger, Stop and Shop, Pathmark, Supermarkets General, Vons, and several major dairy product producers (Kraft, Borden, and Dannon) publicly declared that their companies would not sell or make dairy products treated with the hormone.”
See Schurman and Munro, Fighting, 128-29. Rifkin’s campaign against rBGH took place mostly from 1986 to 1992. Note that similar tactics worked in Europe: “One of the anti-GMO movement’s chief strategies after 1995 involved organizing pressure campaigns on European food retailers. In March 1998, these supermarket campaigns began to pay off when a maverick frozen food company named Iceland Foods agreed to renounce the use of GM ingredients in its store brand products. Over the next year and half, dozens of other European food companies followed Iceland’s lead and moved to clear their own shelves and brands of GM food.” See Schurman and Munro, Fighting, 108-109.
[17] Ibid.
[18] Schurman and Munro, Fighting, xi-xii.
[19] Ibid., xii.
[20] Note that European attitudes on GM food were in most cases moving from an already-suspicious baseline. “[C]onsumer acceptance of green biotechnology in the European Union was already rather low before 1996, when GE foods first appeared on the EU market and extensive NGO campaigns began. For example, from 1991 on surveys indicate rapidly declining optimism about biotechnology among EU respondents.” Thomas Bernauer, Genes, Trade, and Regulation: The Seeds of Conflict in Food Biotechnology (Princeton: Princeton University Press, 2016), 74-75.
[21] Same-Sex Marriage and Gay Rights: A Shift in Americans’ Attitudes, The Associated Press-NORC Center for Public Affairs Research, May 5 2015, http://www.apnorc.org/projects/Pages/HTML%20Reports/same-sex-marriage-and-gay-rights-a-shift-in-americans-attitudes0305-8272.aspx.
[22] George Gaskell, et al. “Worlds Apart? The Reception of Genetically Modified Foods in Europe and the U.S.” Science 285 (1999): 384-86.
[23] Ibid., 385.
[24] For example, self-driving cars received a great deal of press attention coverage from 2017 to 2018, much of it positive (this period predates the 2018 self-driving deaths associated with Uber and Tesla). American Automobile Association polling spanning that period indicated that “63 percent of U.S. drivers report feeling afraid to ride in a fully self-driving vehicle [in early 2018], a significant decrease from 78 percent in early 2017.” (The 2018 poll was conducted in January, well before an Uber test vehicle killed a pedestrian in Arizona.) More Americans Willing to Ride in Fully Self-Driving Cars, AAA NewsRoom, January 24, 2018, http://newsroom.aaa.com/2018/01/americans-willing-ride-fully-self-driving-cars/.
[25] These surveys sampled 1,000 adults via phone and have a 95% confidence interval of +/- 3 percentage points, and so the smaller fluctuations observed are just outside the margin of error. Note the question omits the term “GMO,” possibly contributing to broadly more favorable results for GM foods. The polls do, however, use the phrase “modified by biotechnology.” Full phrasing: “All things being equal, how likely would you be to buy a variety of produce, like tomatoes or potatoes, if it had been modified by biotechnology to be protected from insect damage and required fewer pesticide applications? Would you be very likely, somewhat likely, not too likely, or not at all likely to buy these items?”
[26] In accounts of biotechnology’s ascent, the vision, exuberance, and confidence of experts at the time is striking. It resembles the discourse around clean meat today. (For selections, see appendix two.)
[27] Schurman and Munro, Fighting, 116.
[28] Schurman and Munro, Fighting, 183.
[29] Bernauer, Genes, 174.
[30] Herring, “Political Economy,” 8.13. Citations omitted.
[31] Schurman and Munro, Fighting, xxiii-xxv.
[32] Ibid., xxiv.
[33] Ibid., xxii-xxiv.
[34] See the section on broader social concerns.
[35] “The European Union has moved from a situation of no regulation of agricultural biotechnology in the early- to mid-1980s to very strict approval regulation for GE crops, foods, and feeds, and to increasingly strict and harmonized labeling requirements.” Bernauer, Genes, Trade, and Regulation, 54.
[36] “Europe’s second-highest court on Friday overturned a decision by the European Commission to allow the cultivation and sale of a genetically modified potato developed by German chemicals group BASF…. The General Court of the European Union said the Commission had failed to follow the bloc’s rules when approving the Amflora potato, which is genetically modified to produce extra starch for use in the paper industry.” “EU court annuls approval of BASF’s Amflora GMO potato,” Reuters, December 13, 2013, https://www.reuters.com/article/eu-gmo-potato/eu-court-annuls-approval-of-basfs-amflora-gmo-potato-idUSL6N0JS1TH20131213.
[37] “[M]arket closure [in Europe]... occurred in two mutually reinforcing ways. One involved the food retailing industry’s decision to go GMO free, and the other involved a political shift at the level of the European Union, ultimately resulting in a moratorium on new GM crop approvals. Anti-biotechnology activists played a crucial role in both of these processes.” Schurman and Munro, Fighting, 107.
[38] Schurman and Munro, Fighting, 109.
[39] “Reflecting its US-centric and supremely confident attitude, Monsanto had arrived on the continent without so much as a phone call to any major food processing and retail company in Europe, even though the company was thoroughly dependent on these firms to buy and sell its products. This dependence, however, was not mutual, because European food retailers and manufacturers could survive perfectly well without getting involved in the GM food trade. (After the mid-1990s, in fact, they were likely to be better off if they stayed out of the GM food business, since their customers were telling them that they did not want GM food.) The failure of the US biotechnology industry to persuade European retailers to “buy in” on the technology turned out to be a serious error in judgment. As one activist sardonically noted, ‘They assumed that they could just manage it.’” Schurman and Munro, Fighting, 111.
[40] The “response by downstream producers to consumer and NGO demands has been facilitated by high concentration in the food retail sector in the European Union and low concentration in the farm and grain-handling sectors. Here lies one of the key differences between the European Union and the United States in industrial structure. This difference helps in accounting for the weak collective action capacity of pro-biotech producers in the European Union but not in the United States.
“Available indices of market concentration suggest that in 1996, when the controversy over biotech food began, the top 20 retail firms in the European Union controlled around 40–60 percent of the EU market. In several EU countries, market concentration was as high as 60–80 percent. Dominance by one firm or a duopoly was (and still is) a dominant pattern. The available data also suggests that concentration in the US retail food market was lower at that point in time.
“Concentration of the retail sector in the hands of fewer firms in the European Union, and particularly their efficient organization through Eurocommerce, a Brussels-based association of the European retail and wholesale sector, has made it easier for European downstream producers to switch position in line with consumer and NGO demands.” Bernauer, Genes, 86-88.
[41] “The supermarket sector had… come to be dominated by a relatively small number of large and influential firms. Competition within this sector was extremely fierce and rested on these firms’ abilities to establish themselves as purveyors of competitive pricing and quality, which was captured in their house brands. In this highly competitive environment, any significant customer defection posed a serious threat. This made supermarkets an excellent target for activist attacks, particularly when those attacks… question[ed] the quality of a firm’s store brand.” Schurman and Munro, Fighting, 109. Additionally, food retail firms tend to be highly visible, which meant that questions of reputational damage became more important than they otherwise might have, intensifying concerns around perceived food quality and safety.
[42] “[T]he food industry as a whole became extremely vulnerable to the perception that the food it was selling was unsafe. Food retailers and processors were thus inclined to do whatever was necessary to maintain the public’s confidence and trust, because their company’s brand names and reputations were on the line. As the president and CEO of Novartis’s Gerber baby food division noted in explaining why his company decided to go GMO free, ‘I have got to listen to my customers. So, if there is an issue, or even an inkling of an issue, I am going to make amends. We have to act pre-emptively.” Schurman and Munro, Fighting, 111.
[43] Schurman and Munro, Fighting, 96. This sort of objection to life patenting would play a role in GM debates around the world. See also the section about intellectual property and patents.
[44] Ibid., 97.
[45] Ibid.
[46] “[N]ot long after the first few [GMO] petitions arrived at the European Commission and were approved by the European Council and Parliament, activists recognized the myriad possibilities of disrupting the approval process and began working with their allies among the member states.” Schurman and Munro, Fighting, 112-113.
[47] Ibid., 113.
[48] “[M]ore and more petitions for GMO approval became logjammed by requests for more risk-related data. By 1998, the approval system was so bogged down that no new applications were getting through.” Schurman and Munro, Fighting, 114.
[49] Although tremors of France’s conversion “can be dated as early as February 12, 1997, when Prime Minister Alain Juppé suddenly decided that cultivation of this GM maize could not be authorized.” Marcel Kuntz, “The GMO case in France: Politics, lawlessness and postmodernism,” GM Crops & Food 5, no. 3 (2014): 163-169.
[50] Schurman and Munro, Fighting, 115.
[51] “Whereas the biotechnology industry and many EU government officials… strongly preferred a system that would regulate biotechnology on the basis of the food, medicine, and other products the technology produced (a ‘product-based system’), opponents of the technology, as well as many environmental officials, wanted a process-based system that would apply to all genetically modified organisms, whatever their final form and use. Under the former approach, some GMOs might not be subjected to any regulatory process or requirements if they were considered to be ‘substantially equivalent’ to foods or crops currently on the market; under the latter approach, every use of genetic engineering techniques would be regulated by a new set of policies designed specifically for this purpose.” Schurman and Munro, Fighting, 98.
[52] Ibid., 84.
[53] Ibid.
[54] Ibid., 118.
[55] Collected by Ronald Herring and Robert Paarlberg in “The Political Economy of Biotechnology,” Annual Review of Resource Economics (2016): 8.1-8.20, who are also relying on Rachel Schurman, “Fighting ‘Frankenfoods’: industry opportunity structures and the efficacy of the anti-biotech movement in Western Europe,” Social Problems 51, no. 2 (2004): 243–68. In-text citations omitted.
[56] Sheila Jasanoff, Designs of Nature: Science and Democracy in Europe and the United States (Princeton, NJ: Princeton University Press, 2005), 40, in addition to Herring and Paarlberg, “Political Economy,” 8.10.
[57] Herring, “Political Economy,” 8.10.
[58] Charles, Lords, 28. Further corroborated by New York Times reporting from 2001:
“In late 1986, four executives of the Monsanto Company, the leader in agricultural biotechnology, paid a visit to Vice President George Bush at the White House to make an unusual pitch.
“Although the Reagan administration had been championing deregulation across multiple industries, Monsanto had a different idea: the company wanted its new technology, genetically modified food, to be governed by rules issued in Washington -- and wanted the White House to champion the idea.
“‘There were no products at the time,’ Leonard Guarraia, a former Monsanto executive who attended the Bush meeting, recalled in a recent interview. ‘But we bugged him for regulation. We told him that we have to be regulated.’
“Government guidelines, the executives reasoned, would reassure a public that was growing skittish about the safety of this radical new science. Without such controls, they feared, consumers might become so wary they could doom the multibillion-dollar gamble that the industry was taking in its efforts to redesign plants using genes from other organisms -- including other species.”
See Kurt Eichenwald, “Redesigning Nature: Hard Lessons Learned; Biotechnology Food: From the Lab to a Debacle,” New York Times, January 25, 2001.
[59] Eichenwald, “Redesigning.”
[60] Bernauer, Genes, 11-13.
[61] Ibid., 13.
[62] Schurman and Munro, Fighting, 120.
[63] “Europeans trust regulatory agencies and politicians much less than North Americans. Similarly, while US consumers trust scientific associations and the FDA as sources of information on food safety issues, Europeans express more trust in consumer and environmental groups.” Bernauer, Genes, 76-77.
[64] Bernauer, Genes, 10-13.
[65] Herring, “Political Economy,” 8.12, citing G. Hofstede, Culture’s Consequences: Comparing Values, Behaviors, Institutions, and Organizations Across Nations (London: Sage, 2001).
[66] E.g., the National Farmers Union in the US released the following statement supporting GMO labeling in abstract but opposing a specific 2016 bill to establish mandatory federal labeling:
“Many of our members have chosen to incorporate genetically modified organisms (GMOs) into their production methods, while others have made different choices. The rights of GMO and non-GMO producers should be respected as equal while public concerns about GMOs are evaluated by federal agencies.
“NFU also values consumer rights, including the ability of consumers to have access to as much pertinent information as they want to know about their food. We support mandatory labeling of foods derived from genetically engineered plants, although we do not have policy on what such labeling should look like. As such, NFU opposes the proposed GMO labeling bill in its current form.”
See National Farmers Union, NFU Statement on Proposed Senate GMO Labeling Bill, February 23, 2016, https://nfu.org/2016/02/23/nfu-statement-on-proposed-senate-gmo-labeling-bill/.
[67] Kuntz, “GMO case in France,” 163.
[68] Schurman and Munro, Fighting, 118.
[69] “Gerber and H.J. Heinz... announced in 1999 that they would soon switch to non-GM ingredients—not because of any new evidence that transgenic ingredients were unsafe, but out of fear of a Greenpeace-led boycott. Frito-Lay… followed suit, announcing that it would no longer use GM corn.” Robert Paarlberg, “The Global Food Fight,” Foreign Affairs 79, no. 3 (2000): 24-38.
[70] “The biotechnology industry actually began not with large multinational corporations with but with a group of small, specialized firms called ‘new biotechnology firms’ or ‘biotech startups.’ These firms first came onto the scene in the mid-1970s and grew rapidly in number over the next half dozen or so years.” Schurman and Munro, Fighting, 18-19.
[71] Ibid.
[72] Schurman and Munro, Fighting, xi.
[73] “These young genetic engineers [at Monsanto and other biotech startups] did believe that their work would be good for the planet, possibly making it easier to grow food or reducing agriculture’s dependence on chemicals. Some of them, working inside chemical companies, often saw themselves as ‘green’ revolutionaries fighting against the entrenched power of the chemists… They’d seen DDT banned and Earth Day celebrated. Chemicals represented a dirty and regrettable past, and biology was the savior.
“At Monsanto those views ‘came from the very top,’ says Pam Marrone, a researcher at Monsanto during the late 1980s. ‘I remember having lunch with [then-CEO] Dick Mahoney and him saying, “Because of parathion [a particularly hazardous insecticide], I don’t ever want to be in chemicals again. And that’s why we’re in biotechnology.”’”
Charles, Harvest, 24-25.
[74] Schurman and Munro, Fighting, 38-39.
[75] Sylvie Bonny, “Why are most European opposed to GMOs? Factors explaining rejection in France and Europe,” Electronic Journal of Biotechnology 6, no. 1 (2003): 64.
[76] Nina Fedoroff and Nancy Marie Brown, Mendel in the Kitchen (Washington, D.C.: Joseph Henry Press, 2004), 8.
[77] Quoted in Fedoroff, 287.
[78] Bernauer, Genes, 181.
[79] “The cultivation of genetically modified (GM) crops with new transgenic traits such as herbicide tolerance (HT) is spreading fast in cotton growing states even though no license or approval has been granted by authorities such as GEAC or ICAR for growing them in India… Farmers are swayed by the multiple benefits of these GM varieties, which are being sold illegally, as they offer the twin advantage of bollworm resistance and herbicide tolerance. In comparison, the approved Bt variety (Bollgard I and Bollgard II) is only bollworm-resistant… The new GM varieties are being sold at half the price of approved hybrid cotton seeds by the grey market players, who seem to be outsmarting regulatory officials by operating directly in remote parts without any valid licenses.” B Dasarath Reddy, “Grey market corners new GM hybrids as farmers look beyond Bt cotton,” Business Standard, July 24, 2017, http://www.business-standard.com/article/economy-policy/grey-market-corners-new-gm-hybrids-as-farmers-look-beyond-bt-cotton-117072400399_1.html. Hyperlinks sic.
[80] Fedoroff, Mendel, 312.
[81] Christophe Bonneuil, “Disentrenching Experiment: The Construction of GM--Crop Field Trials As a Social Problem,” Science, Technology & Human Values 33 (2008): 225.
[82] Ibid., 217.
[83] Charles, Harvest, 168.
[84] See Gaskell, “Worlds Apart,” 384-385.
[85] Bonneuil, 219.
[86] Schurman and Munro, Fighting, 18-20.
[87] “A second idea that made ‘common sense’ to the business staffs of these companies (and one that they constantly sought to impress upon the scientists in their midst) related to the kind of products their companies should focus on developing…. [T]he products of greatest interest were those that offered the largest market potential. Market potential was typically defined in terms of sales volume…. A market focus meant that while some sorts of research were highly appealing to the executives of these large companies, others were considered a waste of time because they did not anticipate significant demand. It was this basic business reality that explained why companies aggressively sought to develop herbicide-resistant plants and crops into which the naturally occurring insecticide Bacillus thuringiensis could be engineered, and why they generally avoided pursuing others that might have had more value from a societal perspective, such as nutritionally enhanced cereal crops and drought-resistant crops cultivated mainly by farmers in the global South.” Ibid., 38.
[88] Schurman, 105.
[89] Charles, Harvest, 168-169.
[90] Ibid., 170.
[91] “Even as Monsanto was assembling its outreach strategy, other documents show that it was making strides toward what former executives now acknowledge was a major strategic blunder. The company was preparing to introduce to farmers the first product from its biotechnology program: a growth hormone produced in genetically altered bacteria. Some on the strategy committee pushed for marketing a porcine hormone that would produce leaner and bigger hogs.
“But, simply because the product was further along in development, the company decided to go forward with a bovine growth hormone, which improves milk production in cows -- despite vociferous objections of executives who feared that tinkering with a product consumed by children would ignite a national outcry.
“‘It was not a wise choice to go out with that product first,’ Mr. Harbison acknowledged. ‘It was a mistake.’
“Scientists who watched the events remain stunned by Monsanto's decisions.
“‘I don't think they really thought through the whole darn thing,’ Dr. Virginia Walbot, a professor of biological sciences at Stanford University, said of Monsanto's decision to market products that benefited farmers rather than general consumers. ‘The way Thomas Edison demonstrated how great electricity was was by providing lights for the first nighttime baseball game. People were in awe. What if he had decided to demonstrate the electric chair instead? And what if his second product had been the electric cattle prod? Would we have electricity today?’
“The decision touched off a furor. Jeremy Rifkin, director of the Foundation on Economic Trends, an opponent of biotechnology, joined with family-farm groups worried about price declines and other organizations in a national campaign to keep the Monsanto hormone out of the marketplace. Some supermarket chains shunned the idea; several dairy states moved to ban it. The first step toward the shopping cart brought only bad news.
See Eichenwald, “Redesigning Nature.”
[92] Ibid.
[93] Charles, Harvest, 24-25.
[94] Ibid., 32-33.
[95] Charles, Harvest, 25.
[96] Shapiro, Clean Meat, 213-14.
[97] Schurman and Munro, Fighting, 20.
[98] Ibid., 36.
[99] “In the United States disapproval is strongest among people over sixty-four, among women, and among people with low levels of education. An identical pattern emerges in Europe. Americans with postgraduate degrees are among those most likely to approve of GMOs. Approval also correlates with high income, but not independent from educational attainment.” Paarlberg, Starved for Science, 25.
[100] Bernauer, Genes, 169.
[101] Ron Herring, “Epistemic brokerage in the bio-property narrative: contributions to explaining opposition to transgenic technologies in agriculture,” New Biotechnology 27, no. 5 (2010): 614-622.
[102] “The first step forward, then, is to split up the concept of GMO, to think of it as the product of a particular juncture in history. That juncture combined real concerns of unknown risks of new technology and demonstrably faulty state regulation. But the science has moved on. Vital questions about crops and interests for the future involve more splitting and less lumping: what traits, what cultivars, which genetic events, where and under what conditions for what developmental purposes? Only with this knowledge can we devise priorities and steering mechanisms as aspirational and precise as the potentials of the technology.” Ron Herring, “Opposition to transgenic technologies: ideology, interests and collective action frames,” Science and Society (2008) 9: 458-63.
[103] Schurman and Munro, Fighting, 104-105.
[104] “When you explain to someone that ‘cultured meat’ is meat produced in a culture instead of in a live animal, inevitably you conjure an image of meat produced in a petri dish. But that’s wrong, of course. Although the process involves petri dishes and laboratories at the earliest stages, clean meat production will happen in the equivalent of giant meat fermenters once it’s at production scale. Growing meat at scale will look like a beer brewery or a greenhouse, not like a laboratory.” Bruce Friedrich, “‘Clean Meat’: The ‘Clean Energy’ of Food,” Good Food Institute, September 6, 2016, http://www.gfi.org/clean-meat-the-clean-energy-of-food.
[105] As of 2014, non-craft breweries accounted for about 88% of US beer consumption. (This percentage was as high as ~96% in the 1990s.) Craft breweries are indeed claiming a greater share of the beer market, but the industry remains quite centralized, especially in light of the $107 billion merger between Anheuser-Busch InBev and SABMiller in 2016. See “Can you imagine a world without Budweiser? We can,” The Conversation, May 4, 2016, https://theconversation.com/can-you-imagine-a-world-without-budweiser-we-can-56791.
[106] Schurman and Munro, Fighting, 104-106.
[107] Schurman and Munro, Fighting, 106.
[108] Robert Paarlberg, “Strengthening NSF Sponsored Agribiotechnology Research,” statement to the U.S. House of Representatives Committee on Science, September 25, 2001, http://www.agbioworld.org/newsletter_wm/index.php?caseid=archive&newsid=1196. Some of the crops Paarlberg mentions, such as Bt cotton in India, eventually won approval.
[109] Paarlberg, Starved for Science, 16-18.
[110] Ibid.
[111] Bernauer, Genes, 77. (A note: these public health scares do coincide with the sharpest and most decisive drop in public support for GMOs, from 1996 to 1999, but establishing anything beyond correlation is difficult given the range of things plausibly influencing public opinion on any complex topic, including biotechnology.)
[112] Kuntz, 162-63. He continues: “To understand the attitude of French politicians, it is necessary to mention the HIV-tainted blood scandal in the country in the mid 80s when hemophiliacs were given blood products known to be contaminated: it not only sparked legitimate emotions because the perpetrators were medical doctors, but also because many considered that the government did not react appropriately (subsequently a former Prime Minister, a Health Minister and a Social Affairs Minister stood trial before a special court). Subsequently,politicians were not willing to take any risk for their own career when a technological risk—even hypothetical and even when scientifically refuted—was subject to media attention.”
[113] “This countercultural world view was informed by the particular generation and historical moment in which these activists came of age, as well as by their personal biographies, that is, the events and experiences that shaped their individual lives. Drawing on their experiences in the tumultuous 1960s, being present at the birth of the environmental, feminist, anti-nuclear, and Non-Aligned movements, and observing some of the more pernicious effects of the North's ‘development project’ on the global South, these [activists] were disposed to look upon these technological developments with a critical eye. They were also deeply suspicious of the motives of big business. Consequently, they were inclined to assess the technology in its socioeconomic context. The ideational and normative elements of this countercultural worldview are readily apparent in the interviews we conducted with anti-biotechnology activists.” Schurman and Munro, Fighting, xv, 10, 89, 100.
[114] Charles, Harvest, 250.
[115] Schurman and Munro, Fighting, 60-61.
[116] Schurman, 106-107.
[117] “There may be reasons to be cautious of advancing even scientifically-sound explanations for why clean meat is safe, especially if these explanations are overly technical. As Hans Mathias Kepplinger notes,
“‘the reduction of the negative side effects of technology—or the risks of technology—does not necessarily lead to a decrease in fears and concern. Instead, even small dangers become the occasion for great concern due to increased interest in remote events and potential incidents. It can therefore hardly be supposed that an increase in safety of nuclear power plants or genetic engineering automatically increases acceptance. Making increased safety a theme of topical interest would presumably rather add to the concern than reduce it, because it brings facts found to be threatening into people’s consciousness without the population being able to understand the arguments.’
“Kepplinger cites as an example water fluoridation experiments in which researchers found that ‘acceptance [of fluoridation] dropped due solely to the subject being made a theme of popular interest. This was still the case even if the arguments in favour of fluoridation were presented in a suitable way.’”
J. Mohorčich, “What can nuclear power teach us about the institutional adoption of clean meat?” Sentience Institute, November 28, 2017, https://www.sentienceinstitute.org/nuclear-power-clean-meat#public-narrative.
Consider also the notion that precautionary regulation can raise more suspicion than it avoids. After the formation of the Recombinant DNA Advisory Committee, scientist Joshua Lederberg worried “that just the act of regulating recombinant DNA research would make people think it was dangerous, whether it was or not. Reflecting on what happened in the following decades, Jim Watson was quite blunt, saying ‘And boy, he was right’” (Fedoroff 143).
Herring agrees, writing that “Risk perceptions were… reinforced by the [1997 EU] labeling regulation” (Herring et al. 8.6). The evidence on this, however does not go beyond expert opinions.
[118] “In the early stages of the DNA debate (1973-1975), policy-making was largely initiated and controlled by scientists and administrators involved in biological research, that is, by researchers with little experience or expertise in public participation. Their role was a reactive one, a succession of stopgaps, and finally a painful accommodation to increasingly ‘foreign’ pieces of politics inserted in their normally private decision-making machinery.” Rae Goodell, “Public Involvement in the DNA Controversy: The Case of Cambridge, Massachusetts,” Science, Technology, & Human Values 4, no. 27 (1979): 36-43.
[119] Bernauer, Genes, 40.
[120] “This bill… prohibits misrepresenting a product as meat that is not derived from harvested production livestock or poultry…. PROPONENTS: Supporters say that the livestock industry has spent a lot of time and money educating consumers and promoting its products. This bill would keep manufacturers of plant-based proteins from calling their products meat and benefiting from the work of the livestock industry. Testifying for the bill were Representative Knight; Missouri Soybean Association; Missouri Pork Association; Missouri Corn Growers Association; Missouri Farm Bureau; Missouri Poultry Federation; and Andy McCaskill, Missouri Cattleman’s [sic] Association. OPPONENTS: There was no opposition voiced to the committee.” See Missouri State House, HB2607, 2018, https://house.mo.gov/billtracking/bills181/sumpdf/HB2607C.pdf. (Later joined by Senate Bill 627, which includes similar language.)
[121] US Cattlemen’s Association, “PETITION FOR THE IMPOSITION OF BEEF AND MEAT LABELING REQUIREMENTS: TO EXCLUDE PRODUCTS NOT DERIVED DIRECTLY FROM ANIMALS RAISED AND SLAUGHTERED FROM THE DEFINITION OF ‘BEEF’ AND ‘MEAT,’” February 9, 2018, https://www.fsis.usda.gov/wps/wcm/connect/e4749f95-e79a-4ba5-883b-394c8bdc97a3/18-01-Petition-US-Cattlement-Association020918.pdf?MOD=AJPERES.
[122] Bernauer, Genes, 78.
[123] See The Good Food Institute, “RE: U.S. Cattlemen’s Association Petition to Restrict Beef and Meat Terms on Food Labels,” response to FSIS-2018-0016, April 17, 2018, http://www.gfi.org/images/uploads/2018/04/GFIetal-Comment-FSIS-2018-0016.pdf; Zach Weissmueller, “Lab-Grown Meat Is Coming to Your Supermarket. Ranchers Are Fighting Back,” April 26, 2018, https://reason.com/reasontv/2018/04/26/just-lab-grown-clean-meat-tetrick; and Elaine Watson, “Where's the beef? The cell-cultured variety is still 'meat,' says attorney as cattlemen petition USDA over clean meat labeling,” March 3, 2018, https://www.foodnavigator-usa.com/Article/2018/03/03/Where-s-the-beef-The-cell-cultured-variety-is-still-meat-says-attorney-as-cattlemen-petition-USDA-over-clean-meat-labeling.
[124] Gaskell, “Biotechnology and the European public,” 2000. For example, Gaskell writes that “[a]mong Europeans with well-formed attitudes genetic testing remains at over 90% support in 1999, with GM medicines falling marginally from 91% in 1996 to 87% in 1999. In contrast, a moderate decline in support for the production of GM crops and a sharp decline in support for GM foods have taken place. In 1996, for example, 61% of Europeans opting for one of the three common logics were either supporters or risk-tolerant supporters of GM foods, and 39% were opponents; but three years later, 47% were supporters or risk-tolerant supporters, and an overall majority of 53% were opponents of this technology. Overall, it appears that the secular trend in declining optimism about biotechnology reflects growing opposition to specific applications and not to wholesale rejection of modern biotechnology.”
[125] Herring, “Political Economy,” 8.8.
[126] Ibid., 8.8, citing Chris Fife-Schaw and Gene Rowe, who write: “In the study of discrepancies between expert and lay perceptions of hazards, the ‘psychometric approach’ (e.g. Fischhoff et al., 1978; Kraus and Slovic, 1988) has proven informative. In contrast to expert assessments based on actuarial data, lay perceptions of risk involve factors in addition to the likelihood and seriousness of harm from a hazard, such as the amount of control individuals may have over exposure and the degree to which the hazards are identifiable and understood.” Chris Fife-Schaw and Gene Rowe, “Extending the application of the psychometric approach for assessing public perceptions of food risk: some methodological considerations,” Journal of Risk Research 3, no. 2 (2000): 167–179.
[127] Language and ideas here from an exchange with Jacy Reese, May 7.
[128] Bernauer, Genes, 1.
[129] Ibid., 1-2.
[130] Ibid., 2.
[131] Ibid., 3.
[132] Bernauer, Genes, 19.
[133] Ibid., 20.
[134] Ibid, 20-21.
[135] It’s possible that JUST’s work on malnutrition in Africa may indicate a movement toward broader, more mid-stage considerations. See Caitlin Dewey, “The Silicon Valley food start-up best known for its vegan mayo thinks it can cure malnutrition in Africa,” The Washington Post, February 23, 2018, https://www.washingtonpost.com/news/wonk/wp/2018/02/23/the-silicon-valley-food-start-up-best-known-for-its-vegan-mayo-thinks-it-can-cure-malnutrition-in-africa/?utm_term=.c4b223b50904.
[136] Identity preservation or IP refers to a system in which certain characteristics of a commodity are tracked and remain visible as the commodity moves through a supply chain. E.g., a shipment of corn could be marked as GM or non-GM in an IP system. That status would remain available to handlers, food processors, retailers, and so forth as the corn made its way to consumers.
[137] All points from Bernauer, Genes, 41.
[138] Consumer Reports, “Why Grass-Fed Beef Costs More,” August 24, 2015, https://www.consumerreports.org/cro/magazine/2015/08/why-grass-fed-beef-costs-more/index.htm.
[139] The proposed language: “For fiscal year 2018 and hereafter, the [Agriculture] Secretary shall regulate products made from cells of amenable species of livestock, as defined in the Federal Meat Inspection Act, or poultry, as defined in the Poultry Products Inspection act, grown under controlled conditions for use as human food, and shall issue regulations prescribing the type and frequency of inspection required for the manufacture and processing of such products, as well as other requirements necessary to prevent the adulteration and misbranding of these products.” See “A Bill Making appropriations for Agriculture, Rural Development, Food and Drug Administration, and Related Agencies programs for the fiscal year ending September 30, 2019, and for other purposes,” 2018, https://docs.house.gov/meetings/AP/AP01/20180509/108287/BILLS-115HR-SC-AP-FY2019-Agriculture-SubcommitteeDraft.pdf, 90. (The bill eventually failed to pass, but as of this writing similar language remains a possibility in future legislation.)
[140] Shapiro, Clean Meat, 213.
[141] Charles, Lords, 28. Further corroborated by New York Times reporting from 2001:
“In late 1986, four executives of the Monsanto Company, the leader in agricultural biotechnology, paid a visit to Vice President George Bush at the White House to make an unusual pitch.
“Although the Reagan administration had been championing deregulation across multiple industries, Monsanto had a different idea: the company wanted its new technology, genetically modified food, to be governed by rules issued in Washington -- and wanted the White House to champion the idea.
“‘There were no products at the time,’ Leonard Guarraia, a former Monsanto executive who attended the Bush meeting, recalled in a recent interview. ‘But we bugged him for regulation. We told him that we have to be regulated.’
“Government guidelines, the executives reasoned, would reassure a public that was growing skittish about the safety of this radical new science. Without such controls, they feared, consumers might become so wary they could doom the multibillion-dollar gamble that the industry was taking in its efforts to redesign plants using genes from other organisms -- including other species.”
See Kurt Eichenwald, “Redesigning Nature: Hard Lessons Learned; Biotechnology Food: From the Lab to a Debacle,” New York Times, January 25, 2001.
[142] Eichenwald, “Redesigning.”
[143] (Optimistic or pessimistic, it’s worth noting—see next bullet.)
[144] “One of Monsanto's arguments is that when farmers save seed from a crop grown from patented seed and then use that seed for another crop, they are illegally replicating, or ‘making,’ Monsanto's proprietary seeds instead of legally ‘using’ the seeds by planting them only one time and purchasing more seeds for each subsequent planting.
“This logic is troubling to many who point out that it is the nature of seeds and all living things, whether patented or not, to replicate. Monsanto’s claim that it has rights over a self-replicating natural product should raise concern. Seeds, unlike computer chips, for example, are essential to life. If people are denied a computer chip, they don't go hungry. If people are denied seeds, the potential consequences are much more threatening.
“Although Monsanto and other agrochemical companies assert that they need the current patent system to invent better seeds, the counterargument is that splicing an already existing gene or other DNA into a plant and thereby transferring a new trait to that plant is not a novel invention. A soybean, for example, has more than 46,000 genes. Properties of these genes are the product of centuries of plant breeding and should not, many argue, become the product of a corporation. Instead, these genes should remain in the public domain.”
See George Kimbrell and Debbie Barker, “Monsanto, the court and the seeds of dissent,” Los Angeles Times, February 19, 2013, http://articles.latimes.com/2013/feb/19/opinion/la-oe-kimbrell-monsanto-supreme-court-seed-20130219.
[145] A. W. Sorenson et al., “Impact of 'Mad Cow Disease' publicity on trends in meat and total vitamin A consumption in Geneva between 1993 and 2000,” European Journal of Clinical Nutrition 57, no. 1 (2003): 177-85.
[146] Bernauer, Genes, 40.
[147] This and next Wirthlin Group, sample size: 1,036, March 14-16, 1994.
[148] This and next Hoban, “Consumer acceptance.”
[149] This and next Susanna Horning Priest / Public Policy Research Institute, Texas A&M University, April 10 - May 3, 2000. Sample size: 1,002.
[150] This and next ABC News, June 2001 and 2003. Sample size: 1,024
[151] Mellman Group/Public Opinion Strategies for the Pew Initiative on Food and Biotechnology, January 22-28, 2001. Sample size: 1,001.
[152] CBS News, December 17-19, 1999. Sample size: 1,026.
[153] Next five are Gallup, except fourth in the sequence (2002), which is Harris Interactive/Chicago Council on Foreign Relations (but same phrasing, sample size: 3,262). All five aggregate strong + moderate responses.
[154] Harris Interactive, June 8-12, 2000. Sample size: 1,015.
[155] This and next Angus Reid, September 1998 and February 2000. Sample size: ~1,000.
[156] Pew Research, “The New Food Fights: U.S. Public Divides Over Food Science,” December 1, 2016, http://assets.pewresearch.org/wp-content/uploads/sites/14/2016/12/19170147/PS_2016.12.01_Food-Science_FINAL.pdf. Sample size: 1,480.